The CatSper channel: a polymodal chemosensor in human sperm
- PMID: 22354039
- PMCID: PMC3321208
- DOI: 10.1038/emboj.2012.30
The CatSper channel: a polymodal chemosensor in human sperm
Abstract
The sperm-specific CatSper channel controls the intracellular Ca(2+) concentration ([Ca(2+)](i)) and, thereby, the swimming behaviour of sperm. In humans, CatSper is directly activated by progesterone and prostaglandins-female factors that stimulate Ca(2+) influx. Other factors including neurotransmitters, chemokines, and odorants also affect sperm function by changing [Ca(2+)](i). Several ligands, notably odorants, have been proposed to control Ca(2+) entry and motility via G protein-coupled receptors (GPCRs) and cAMP-signalling pathways. Here, we show that odorants directly activate CatSper without involving GPCRs and cAMP. Moreover, membrane-permeable analogues of cyclic nucleotides that have been frequently used to study cAMP-mediated Ca(2+) signalling also activate CatSper directly via an extracellular site. Thus, CatSper or associated protein(s) harbour promiscuous binding sites that can host various ligands. These results contest current concepts of Ca(2+) signalling by GPCR and cAMP in mammalian sperm: ligands thought to activate metabotropic pathways, in fact, act via a common ionotropic mechanism. We propose that the CatSper channel complex serves as a polymodal sensor for multiple chemical cues that assist sperm during their voyage across the female genital tract.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
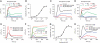

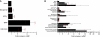
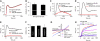





Comment in
-
Sperm are promiscuous and CatSper is to blame….EMBO J. 2012 Apr 4;31(7):1624-6. doi: 10.1038/emboj.2012.62. Epub 2012 Mar 13. EMBO J. 2012. PMID: 22415366 Free PMC article.
References
-
- Adeoya-Osiguwa SA, Dudley RK, Hosseini R, Fraser LR (1998) FPP modulates mammalian sperm function via TCP-11 and the adenylyl cyclase/cAMP pathway. Mol Reprod Dev 51: 468–476 - PubMed
-
- Adeoya-Osiguwa SA, Fraser LR (2003) Calcitonin acts as a first messenger to regulate adenylyl cyclase/cAMP and mammalian sperm function. Mol Reprod Dev 65: 228–236 - PubMed
-
- Aitken RJ, Kelly RW (1985) Analysis of the direct effects of prostaglandins on human sperm function. J Reprod Fertil 73: 139–146 - PubMed
-
- Aitken RJ, Mattei A, Irvine S (1986) Paradoxical stimulation of human sperm motility by 2-deoxyadenosine. J Reprod Fertil 78: 515–527 - PubMed
-
- Aman TK, Shen RY, Haj-Dahmane S (2007) D2-like dopamine receptors depolarize dorsal raphe serotonin neurons through the activation of nonselective cationic conductance. J Pharmacol Exp Ther 320: 376–385 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

