Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency
- PMID: 22354167
- PMCID: PMC3287233
- DOI: 10.1172/JCI61014
Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency
Abstract
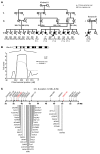
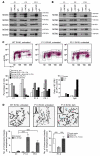
Natural killer (NK) cells are circulating cytotoxic lymphocytes that exert potent and nonredundant antiviral activity and antitumoral activity in the mouse; however, their function in host defense in humans remains unclear. Here, we investigated 6 related patients with autosomal recessive growth retardation, adrenal insufficiency, and a selective NK cell deficiency characterized by a lack of the CD56(dim) NK subset. Using linkage analysis and fine mapping, we identified the disease-causing gene, MCM4, which encodes a component of the MCM2-7 helicase complex required for DNA replication. A splice-site mutation in the patients produced a frameshift, but the mutation was hypomorphic due to the creation of two new translation initiation methionine codons downstream of the premature termination codon. The patients' fibroblasts exhibited genomic instability, which was rescued by expression of WT MCM4. These data indicate that the patients' growth retardation and adrenal insufficiency likely reflect the ubiquitous but heterogeneous impact of the MCM4 mutation in various tissues. In addition, the specific loss of the NK CD56(dim) subset in patients was associated with a lower rate of NK CD56(bright) cell proliferation, and the maturation of NK CD56(bright) cells toward an NK CD56(dim) phenotype was tightly dependent on MCM4-dependent cell division. Thus, partial MCM4 deficiency results in a genetic syndrome of growth retardation with adrenal insufficiency and selective NK deficiency.
Figures



Comment in
-
Unraveling human natural killer cell deficiency.J Clin Invest. 2012 Mar;122(3):798-801. doi: 10.1172/JCI62620. Epub 2012 Feb 22. J Clin Invest. 2012. PMID: 22354165 Free PMC article.
References
-
- French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15(1):45–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

