Peroral ciprofloxacin therapy impairs the generation of a protective immune response in a mouse model for Salmonella enterica serovar Typhimurium diarrhea, while parenteral ceftriaxone therapy does not
- PMID: 22354292
- PMCID: PMC3346637
- DOI: 10.1128/AAC.05819-11
Peroral ciprofloxacin therapy impairs the generation of a protective immune response in a mouse model for Salmonella enterica serovar Typhimurium diarrhea, while parenteral ceftriaxone therapy does not
Abstract
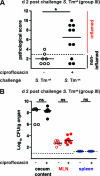
Nontyphoidal Salmonella (NTS) species cause self-limiting diarrhea and sometimes severe disease. Antibiotic treatment is considered only in severe cases and immune-compromised patients. The beneficial effects of antibiotic therapy and the consequences for adaptive immune responses are not well understood. We used a mouse model for Salmonella diarrhea to assess the effects of per os treatment with ciprofloxacin (15 mg/kg of body weight intragastrically 2 times/day, 5 days) or parenteral ceftriaxone (50 mg/kg intraperitoneally, 5 days), two common drugs used in human patients. The therapeutic and adverse effects were assessed with respect to generation of a protective adaptive immune response, fecal pathogen excretion, and the emergence of nonsymptomatic excreters. In the mouse model, both therapies reduced disease severity and reduced the level of fecal shedding. In line with clinical data, in most animals, a rebound of pathogen gut colonization/fecal shedding was observed 2 to 12 days after the end of the treatment. Yet, levels of pathogen shedding and frequency of appearance of nonsymptomatic excreters did not differ from those for untreated controls. Moreover, mice treated intraperitoneally with ceftriaxone developed an adaptive immunity protecting the mice from enteropathy in wild-type Salmonella enterica serovar Typhimurium challenge infections. In contrast, the mice treated intragastrically with ciprofloxacin were not protected. Thus, antibiotic treatment regimens can disrupt the adaptive immune response, but treatment regimens may be optimized in order to preserve the generation of protective immunity. It might be of interest to determine whether this also pertains to human patients. In this case, the mouse model might be a tool for further mechanistic studies.
Figures





Similar articles
-
Results of a 5-year prospective surveillance study of antibiotic resistance among Salmonella enterica isolates and ceftriaxone therapy among children hospitalized for acute diarrhea.Clin Ther. 2002 Oct;24(10):1585-94. doi: 10.1016/s0149-2918(02)80062-5. Clin Ther. 2002. PMID: 12462288
-
Salmonella Typhimurium and Salmonella Enteritidis Infections in Sporadic Diarrhea in Children: Source Tracing and Resistance to Third-Generation Cephalosporins and Ciprofloxacin.Foodborne Pathog Dis. 2019 Apr;16(4):244-255. doi: 10.1089/fpd.2018.2557. Epub 2019 Feb 19. Foodborne Pathog Dis. 2019. PMID: 30779595
-
Antibiotic control of tumor-colonizing Salmonella enterica serovar Typhimurium.Exp Biol Med (Maywood). 2011 Nov;236(11):1282-90. doi: 10.1258/ebm.2011.011111. Epub 2011 Oct 10. Exp Biol Med (Maywood). 2011. PMID: 21987828
-
Epidemiology and Genomics of Invasive Nontyphoidal Salmonella Infections in Kenya.Clin Infect Dis. 2015 Nov 1;61 Suppl 4(Suppl 4):S317-24. doi: 10.1093/cid/civ711. Clin Infect Dis. 2015. PMID: 26449947 Free PMC article. Review.
-
The streptomycin mouse model for Salmonella diarrhea: functional analysis of the microbiota, the pathogen's virulence factors, and the host's mucosal immune response.Immunol Rev. 2012 Jan;245(1):56-83. doi: 10.1111/j.1600-065X.2011.01070.x. Immunol Rev. 2012. PMID: 22168414 Review.
Cited by
-
Salmonella Typhimurium TTSS-2 deficient mig-14 mutant shows attenuation in immunocompromised mice and offers protection against wild-type Salmonella Typhimurium infection.BMC Microbiol. 2013 Oct 22;13:236. doi: 10.1186/1471-2180-13-236. BMC Microbiol. 2013. PMID: 24148706 Free PMC article.
-
Perturbation of the indigenous rat oral microbiome by ciprofloxacin dosing.Mol Oral Microbiol. 2013 Oct;28(5):404-14. doi: 10.1111/omi.12033. Epub 2013 Jul 12. Mol Oral Microbiol. 2013. PMID: 23844936 Free PMC article.
-
Cecum lymph node dendritic cells harbor slow-growing bacteria phenotypically tolerant to antibiotic treatment.PLoS Biol. 2014 Feb 18;12(2):e1001793. doi: 10.1371/journal.pbio.1001793. eCollection 2014 Feb. PLoS Biol. 2014. PMID: 24558351 Free PMC article.
-
Pathogen-targeting glycovesicles as a therapy for salmonellosis.Nat Commun. 2019 Sep 6;10(1):4039. doi: 10.1038/s41467-019-12066-z. Nat Commun. 2019. PMID: 31492864 Free PMC article.
-
Efficacy of cryptdin-2 as an adjunct to antibiotics from various generations against salmonella.Indian J Microbiol. 2014 Sep;54(3):323-8. doi: 10.1007/s12088-014-0463-y. Epub 2014 Mar 21. Indian J Microbiol. 2014. PMID: 24891740 Free PMC article.
References
-
- Ambrose NS, Johnson M, Burdon DW, Keighley MR. 1985. The influence of single dose intravenous antibiotics on faecal flora and emergence of Clostridium difficile. J. Antimicrob. Chemother. 15:319–326 - PubMed
-
- Balfour AE, Lewis R, Ahmed S. 1999. Convalescent excretion of Salmonella enteritidis in infants. J. Infect. 38:24–25 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

