Targeted intracellular delivery of antituberculosis drugs to Mycobacterium tuberculosis-infected macrophages via functionalized mesoporous silica nanoparticles
- PMID: 22354311
- PMCID: PMC3346638
- DOI: 10.1128/AAC.06049-11
Targeted intracellular delivery of antituberculosis drugs to Mycobacterium tuberculosis-infected macrophages via functionalized mesoporous silica nanoparticles
Abstract
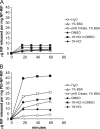
Delivery of antituberculosis drugs by nanoparticles offers potential advantages over free drug, including the potential to target specifically the tissues and cells that are infected by Mycobacterium tuberculosis, thereby simultaneously increasing therapeutic efficacy and decreasing systemic toxicity, and the capacity for prolonged release of drug, thereby allowing less-frequent dosing. We have employed mesoporous silica nanoparticle (MSNP) drug delivery systems either equipped with a polyethyleneimine (PEI) coating to release rifampin or equipped with cyclodextrin-based pH-operated valves that open only at acidic pH to release isoniazid (INH) into M. tuberculosis-infected macrophages. The MSNP are internalized efficiently by human macrophages, traffic to acidified endosomes, and release high concentrations of antituberculosis drugs intracellularly. PEI-coated MSNP show much greater loading of rifampin than uncoated MSNP and much greater efficacy against M. tuberculosis-infected macrophages. MSNP were devoid of cytotoxicity at the particle doses employed for drug delivery. Similarly, we have demonstrated that the isoniazid delivered by MSNP equipped with pH-operated nanovalves kill M. tuberculosis within macrophages significantly more effectively than an equivalent amount of free drug. These data demonstrate that MSNP provide a versatile platform that can be functionalized to optimize the loading and intracellular release of specific drugs for the treatment of tuberculosis.
Figures





Similar articles
-
pH-Responsive Isoniazid-Loaded Nanoparticles Markedly Improve Tuberculosis Treatment in Mice.Small. 2015 Oct;11(38):5066-78. doi: 10.1002/smll.201500937. Epub 2015 Jul 20. Small. 2015. PMID: 26193431 Free PMC article.
-
Antimycobacterial susceptibility evaluation of rifampicin and isoniazid benz-hydrazone in biodegradable polymeric nanoparticles against Mycobacterium tuberculosis H37Rv strain.Int J Nanomedicine. 2018 Jul 23;13:4303-4318. doi: 10.2147/IJN.S163925. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30087562 Free PMC article.
-
Mannosamine-Modified Poly(lactic-co-glycolic acid)-Polyethylene Glycol Nanoparticles for the Targeted Delivery of Rifapentine and Isoniazid in Tuberculosis Therapy.Bioconjug Chem. 2025 May 21;36(5):1021-1033. doi: 10.1021/acs.bioconjchem.5c00062. Epub 2025 Apr 22. Bioconjug Chem. 2025. PMID: 40262736 Free PMC article.
-
The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis.Respir Res. 2001;2(3):164-8. doi: 10.1186/rr54. Epub 2001 Apr 5. Respir Res. 2001. PMID: 11686881 Free PMC article. Review.
-
A Novel Approach in Treatment of Tuberculosis by Targeting Drugs to Infected Macrophages Using Biodegradable Nanoparticles.Appl Biochem Biotechnol. 2018 Jul;185(3):815-821. doi: 10.1007/s12010-018-2695-5. Epub 2018 Jan 19. Appl Biochem Biotechnol. 2018. PMID: 29349532 Review.
Cited by
-
Nanoparticle-Based Inhalation Therapy for Pulmonary Diseases.Curr Drug Metab. 2022;23(11):882-896. doi: 10.2174/1389200223666220803103039. Curr Drug Metab. 2022. PMID: 35927812
-
An ingenious non-spherical mesoporous silica nanoparticle cargo with curcumin induces mitochondria-mediated apoptosis in breast cancer (MCF-7) cells.Oncotarget. 2019 Feb 5;10(11):1193-1208. doi: 10.18632/oncotarget.26623. eCollection 2019 Feb 5. Oncotarget. 2019. PMID: 30838091 Free PMC article.
-
Development of a highly biocompatible antituberculosis nanodelivery formulation based on para-aminosalicylic acid-zinc layered hydroxide nanocomposites.ScientificWorldJournal. 2014;2014:401460. doi: 10.1155/2014/401460. Epub 2014 Jun 23. ScientificWorldJournal. 2014. PMID: 25050392 Free PMC article.
-
Breaking barriers: The potential of nanosystems in antituberculosis therapy.Bioact Mater. 2024 May 17;39:106-134. doi: 10.1016/j.bioactmat.2024.05.013. eCollection 2024 Sep. Bioact Mater. 2024. PMID: 38783925 Free PMC article. Review.
-
Carbohydrate-Conjugated Hollow Oblate Mesoporous Silica Nanoparticles as Nanoantibiotics to Target Mycobacteria.Adv Healthc Mater. 2015 Dec 30;4(18):2797-801. doi: 10.1002/adhm.201500491. Epub 2015 Oct 9. Adv Healthc Mater. 2015. PMID: 26450697 Free PMC article.
References
-
- Ain Q, Sharma S, Khuller GK, Garg SK. 2003. Alginate based oral drug delivery system for tuberculosis: pharmacokinetics and therapeutic effects. J. Antimicrob. Chemother. 51:931–938 - PubMed
-
- Anisimova YV, Gelperina SI, Peloquin CA, Heifets LB. 2000. Nanoparticles as antituberculosis drugs carriers: effect on activity against Mycobacterium tuberculosis in human monocyte-derived macrophages. J. Nanopart. Res. 2:165–171
-
- Barbé C, et al. 2004. Silica particles: a novel drug-delivery system. Adv. Mater. 16:1959–1966
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

