Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial
- PMID: 22354457
- PMCID: PMC3319900
- DOI: 10.1007/s11695-012-0622-3
Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial
Abstract
Background: The mechanisms of amelioration of glycemic control early after laparoscopic Roux-en-Y gastric bypass (LRYGB) or laparoscopic sleeve gastrectomy (LSG) are not fully understood.
Methods: In this prospective, randomized 1-year trial, outcomes of LRYGB and LSG patients were compared, focusing on possibly responsible mechanisms. Twelve patients were randomized to LRYGB and 11 to LSG. These non-diabetic patients were investigated before and 1 week, 3 months, and 12 months after surgery. A standard test meal was given after an overnight fast, and blood samples were collected before, during, and after food intake for hormone profiles (cholecystokinin (CCK), ghrelin, glucagon-like peptide 1 (GLP-1), peptide YY (PYY)).
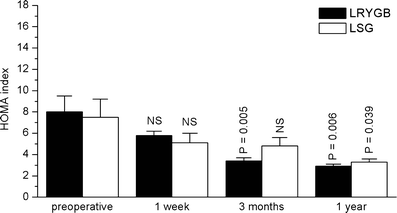
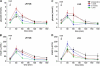
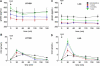
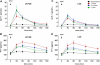
Results: In both groups, body weight and BMI decreased markedly and comparably leading to an identical improvement of abnormal glycemic control (HOMA index). Post-surgery, patients had markedly increased postprandial plasma GLP-1 and PYY levels (p < 0.05) with ensuing improvement in glucose homeostasis. At 12 months, LRYGB ghrelin levels approached preoperative values. The postprandial, physiologic fluctuation returned, however, while LSG ghrelin levels were still markedly attenuated. One year postoperatively, CCK concentrations after test meals increased less in the LRYGB group than they did in the LSG group, with the latter showing significantly higher maximal CCK concentrations (p < 0.012 vs. LRYGB).
Conclusions: Bypassing the foregut is not the only mechanism responsible for improved glucose homeostasis. The balance between foregut (ghrelin, CCK) and hindgut (GLP-1, PYY) hormones is a key to understanding the underlying mechanisms.
Trial registration: ClinicalTrials.gov NCT00356213.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

