Design and synthesis of a bivalent ligand to explore the putative heterodimerization of the mu opioid receptor and the chemokine receptor CCR5
- PMID: 22354464
- PMCID: PMC4374901
- DOI: 10.1039/c2ob06801j
Design and synthesis of a bivalent ligand to explore the putative heterodimerization of the mu opioid receptor and the chemokine receptor CCR5
Abstract
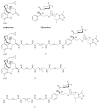
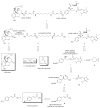
The bivalent ligand approach has been utilized not only to study the underlying mechanism of G protein-coupled receptors dimerization and/or oligomerization, but also to enhance ligand affinity and/or selectivity for potential treatment of a variety of diseases by targeting this process. Substance abuse and addiction have made both the prevention and the treatment of human immunodeficiency virus (HIV) infection more difficult to tackle. Morphine, a mu opioid receptor (MOR) agonist, can accelerate HIV infection through up-regulating the expression of the chemokine receptor CCR5, a well-known co-receptor for HIV invasion to the host cells and this has been extensively studied. Meanwhile, two research groups have described the putative MOR-CCR5 heterodimers in their independent studies. The purpose of this paper is to report the design and synthesis of a bivalent ligand to explore the biological and pharmacological process of the putative MOR-CCR5 dimerization phenomenon. The developed bivalent ligand thus contains two distinct pharmacophores linked through a spacer; ideally one of which will interact with the MOR and the other with the CCR5. Naltrexone and Maraviroc were selected as the pharmacophores to generate such a bivalent probe. The overall reaction route to prepare this bivalent ligand was convergent and efficient, and involved sixteen steps with moderate to good yields. The preliminary biological characterization showed that the bivalent compound 1 retained the pharmacological characteristics of both pharmacophores towards the MOR and the CCR5 respectively with relatively lower binding affinity, which tentatively validated our original molecular design.
Figures

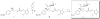

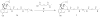

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

