Fluorescein analogues inhibit SecA ATPase: the first sub-micromolar inhibitor of bacterial protein translocation
- PMID: 22354575
- PMCID: PMC3483595
- DOI: 10.1002/cmdc.201100594
Fluorescein analogues inhibit SecA ATPase: the first sub-micromolar inhibitor of bacterial protein translocation
Abstract
SecA is a central component of the general secretion system that is essential for bacterial growth and thus an ideal target for antimicrobial agents. A series of fluorescein analogues were first screened against the ATPase activity using the truncated unregulated SecA catalytic domain. Rose bengal (RB) and erythrosin B (EB) were found to be potent inhibitors SecA with IC(50) values of 0.5 μM and 2 μM, respectively. RB and EB inhibit the catalytic SecA ATPase more effectively than the F(1) F(0) -proton ATPase. We used three assays to test the effect of these compounds on full-length SecA ATPase: in solution (intrinsic ATPase), in membrane preparation, and translocation ATPase. RB and EB show the following trend in terms of IC(50) values: translocation ATPase<membrane ATPase<intrinsic ATPase. Very importantly, the potency of these fluorescein analogues in inhibiting the truncated SecA ATPase correlates with their ability to inhibit the biologically relevant protein translocation activity of SecA. The in vitro translocation of proOmpA precursors into membrane vesicles is strongly inhibited by RB with IC(50) values of approximately 0.25 μM, making RB the most potent inhibitor of SecA ATPase and SecA-dependent protein translocation reported thus far. The ability of these compounds to inhibit SecA also directly translates into antibacterial effects. Our findings show the value of fluorescein analogues as probes for mechanistic studies of SecA functions and for the potential development of new antimicrobial agents with SecA as the target.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



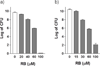

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

