Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children
- PMID: 22354919
- PMCID: PMC3309886
- DOI: 10.1093/cid/cis010
Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children
Abstract
Background: Iron supplementation may increase malaria morbidity and mortality, but the effect of naturally occurring variation in iron status on malaria risk is not well studied.
Methods: A total of 785 Tanzanian children living in an area of intense malaria transmission were enrolled at birth, and intensively monitored for parasitemia and illness including malaria for up to 3 years, with an average of 47 blood smears. We assayed plasma samples collected at routine healthy-child visits, and evaluated the impact of iron deficiency (ID) on future malaria outcomes and mortality.
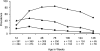
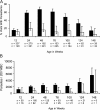
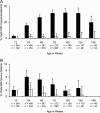
Results: ID at routine, well-child visits significantly decreased the odds of subsequent parasitemia (23% decrease, P < .001) and subsequent severe malaria (38% decrease, P = .04). ID was also associated with 60% lower all-cause mortality (P = .04) and 66% lower malaria-associated mortality (P = .11). When sick visits as well as routine healthy-child visits are included in analyses (average of 3 iron status assays/child), ID reduced the prevalence of parasitemia (6.6-fold), hyperparasitemia (24.0-fold), and severe malaria (4.0-fold) at the time of sample collection (all P < .001).
Conclusions: Malaria risk is influenced by physiologic iron status, and therefore iron supplementation may have adverse effects even among children with ID. Future interventional studies should assess whether treatment for ID coupled with effective malaria control can mitigate the risks of iron supplementation for children in areas of malaria transmission.
Figures
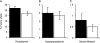
Comment in
-
Food-borne origins of Escherichia coli causing extraintestinal infections.Clin Infect Dis. 2012 Sep;55(5):712-9. doi: 10.1093/cid/cis502. Epub 2012 May 21. Clin Infect Dis. 2012. PMID: 22615330
-
Iron deficiency and malaria mortality: possible implication of invasive bacterial diseases.Clin Infect Dis. 2012 Sep;55(5):748. doi: 10.1093/cid/cis522. Epub 2012 Jun 5. Clin Infect Dis. 2012. PMID: 22670037 No abstract available.
Comment on
-
Iron deficiency and severe Plasmodium falciparum malaria.Clin Infect Dis. 2012 Apr;54(8):1145-7. doi: 10.1093/cid/cis020. Epub 2012 Feb 21. Clin Infect Dis. 2012. PMID: 22354918 Free PMC article. No abstract available.
References
-
- Oski FA, Honig AS, Helu B, Howanitz P. Effect of iron therapy on behavior performance in nonanemic, iron-deficient infants. Pediatrics. 1983;71:877–80. - PubMed
-
- Sheard NF. Iron deficiency and infant development. Nutr Rev. 1994;52:137–40. - PubMed
-
- Sazawal S, Black RE, Ramsan M, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–43. - PubMed
-
- Oppenheimer SJ, Gibson FD, Macfarlane SB, et al. Iron supplementation increases prevalence and effects of malaria: report on clinical studies in Papua New Guinea. Trans R Soc Trop Med Hyg. 1986;80:603–12. - PubMed
-
- Smith AW, Hendrickse RG, Harrison C, Hayes RJ, Greenwood BM. The effects on malaria of treatment of iron-deficiency anaemia with oral iron in Gambian children. Ann Trop Paediatr. 1989;9:17–23. - PubMed

