Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications
- PMID: 22354928
- PMCID: PMC3282805
- DOI: 10.2337/db11-1120
Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications
Abstract
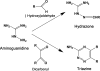
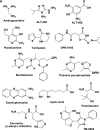
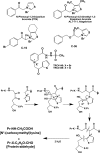
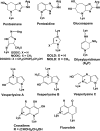
This article outlines evidence that advanced glycation end product (AGE) inhibitors and breakers act primarily as chelators, inhibiting metal-catalyzed oxidation reactions that catalyze AGE formation. We then present evidence that chelation is the most likely mechanism by which ACE inhibitors, angiotensin receptor blockers, and aldose reductase inhibitors inhibit AGE formation in diabetes. Finally, we note several recent studies demonstrating therapeutic benefits of chelators for diabetic cardiovascular and renal disease. We conclude that chronic, low-dose chelation therapy deserves serious consideration as a clinical tool for prevention and treatment of diabetes complications.
Figures

Similar articles
-
Chelation therapy for the management of diabetic complications: a hypothesis and a proposal for clinical laboratory assessment of metal ion homeostasis in plasma.Clin Chem Lab Med. 2014 Jan 1;52(1):69-75. doi: 10.1515/cclm-2012-0881. Clin Chem Lab Med. 2014. PMID: 23612664
-
Chelating activity of advanced glycation end-product inhibitors.J Biol Chem. 2001 Dec 28;276(52):48967-72. doi: 10.1074/jbc.M108196200. Epub 2001 Oct 24. J Biol Chem. 2001. PMID: 11677237
-
Angiotensin II receptor blockers and angiotensin converting enzyme inhibitors: implication of radical scavenging and transition metal chelation in inhibition of advanced glycation end product formation.Arch Biochem Biophys. 2003 Nov 1;419(1):50-4. doi: 10.1016/j.abb.2003.08.010. Arch Biochem Biophys. 2003. PMID: 14568008 Review. No abstract available.
-
Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms.J Am Soc Nephrol. 2002 Oct;13(10):2478-87. doi: 10.1097/01.asn.0000032418.67267.f2. J Am Soc Nephrol. 2002. PMID: 12239236
-
What is the role of direct renin inhibitors in the treatment of the hypertensive diabetic patient?Adv Ther. 2011 Sep;28(9):716-27. doi: 10.1007/s12325-011-0049-6. Epub 2011 Jul 29. Adv Ther. 2011. PMID: 21809181 Review.
Cited by
-
Curcumin, Alone or in Combination with Aminoguanidine, Increases Antioxidant Defenses and Glycation Product Detoxification in Streptozotocin-Diabetic Rats: A Therapeutic Strategy to Mitigate Glycoxidative Stress.Oxid Med Cell Longev. 2020 May 20;2020:1036360. doi: 10.1155/2020/1036360. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32566072 Free PMC article.
-
Diabetes in early pregnancy: getting to the heart of the matter.Diabetes. 2013 Jan;62(1):27-8. doi: 10.2337/db12-1117. Diabetes. 2013. PMID: 23258908 Free PMC article. No abstract available.
-
Advanced Glycation End Products: Potential Mechanism and Therapeutic Target in Cardiovascular Complications under Diabetes.Oxid Med Cell Longev. 2019 Dec 6;2019:9570616. doi: 10.1155/2019/9570616. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31885827 Free PMC article. Review.
-
Lipoxidation in cardiovascular diseases.Redox Biol. 2019 May;23:101119. doi: 10.1016/j.redox.2019.101119. Epub 2019 Feb 25. Redox Biol. 2019. PMID: 30833142 Free PMC article. Review.
-
Uremic Toxicity of Advanced Glycation End Products in CKD.J Am Soc Nephrol. 2016 Feb;27(2):354-70. doi: 10.1681/ASN.2014101047. Epub 2015 Aug 26. J Am Soc Nephrol. 2016. PMID: 26311460 Free PMC article. Review.
References
-
- Goh SY, Cooper ME. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab 2008;93:1143–1152 - PubMed
-
- Tessier FJ. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol Biol (Paris) 2010;58:214–219 - PubMed
-
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 1991;40:405–412 - PubMed
-
- Reddy VP, Beyaz A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov Today 2006;11:646–654 - PubMed
-
- Peyroux J, Sternberg M. Advanced glycation endproducts (AGEs): pharmacological inhibition in diabetes. Pathol Biol (Paris) 2006;54:405–419 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

