Susceptibility of β1 Na+-K+ pump subunit to glutathionylation and oxidative inhibition depends on conformational state of pump
- PMID: 22354969
- PMCID: PMC3320985
- DOI: 10.1074/jbc.M112.340893
Susceptibility of β1 Na+-K+ pump subunit to glutathionylation and oxidative inhibition depends on conformational state of pump
Abstract
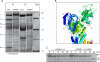
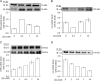
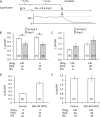
Glutathionylation of cysteine 46 of the β1 subunit of the Na(+)-K(+) pump causes pump inhibition. However, the crystal structure, known in a state analogous to an E2·2K(+)·P(i) configuration, indicates that the side chain of cysteine 46 is exposed to the lipid bulk phase of the membrane and not expected to be accessible to the cytosolic glutathione. We have examined whether glutathionylation depends on the conformational changes in the Na(+)-K(+) pump cycle as described by the Albers-Post scheme. We measured β1 subunit glutathionylation and function of Na(+)-K(+)-ATPase in membrane fragments and in ventricular myocytes. Signals for glutathionylation in Na(+)-K(+)-ATPase-enriched membrane fragments suspended in solutions that preferentially induce E1ATP and E1Na(3) conformations were much larger than signals in solutions that induce the E2 conformation. Ouabain further reduced glutathionylation in E2 and eliminated an increase seen with exposure to the oxidant peroxynitrite (ONOO(-)). Inhibition of Na(+)-K(+)-ATPase activity after exposure to ONOO(-) was greater when the enzyme had been in the E1Na(3) than the E2 conformation. We exposed myocytes to different extracellular K(+) concentrations to vary the membrane potential and hence voltage-dependent conformational poise. K(+) concentrations expected to shift the poise toward E2 species reduced glutathionylation, and ouabain eliminated a ONOO(-)-induced increase. Angiotensin II-induced NADPH oxidase-dependent Na(+)-K(+) pump inhibition was eliminated by conditions expected to shift the poise toward the E2 species. We conclude that susceptibility of the β1 subunit to glutathionylation depends on the conformational poise of the Na(+)-K(+) pump.
Figures





References
-
- Figtree G. A., Liu C. C., Bibert S., Hamilton E. J., Garcia A., White C. N., Chia K. K., Cornelius F., Geering K., Rasmussen H. H. (2009) Reversible oxidative modification. A key mechanism of Na+-K+ pump regulation. Circ. Res. 105, 185–193 - PubMed
-
- Shimon M. B., Goldshleger R., Karlish S. J. (1998) Specific Cu2+-catalyzed oxidative cleavage of Na, K-ATPase at the extracellular surface. J. Biol. Chem. 273, 34190–34195 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

