Distinct roles of Mus81, Yen1, Slx1-Slx4, and Rad1 nucleases in the repair of replication-born double-strand breaks by sister chromatid exchange
- PMID: 22354996
- PMCID: PMC3347241
- DOI: 10.1128/MCB.00111-12
Distinct roles of Mus81, Yen1, Slx1-Slx4, and Rad1 nucleases in the repair of replication-born double-strand breaks by sister chromatid exchange
Erratum in
-
Correction for Muñoz-Galván et al., "Distinct Roles of Mus81, Yen1, Slx1-Slx4, and Rad1 Nucleases in the Repair of Replication-Born Double-Strand Breaks by Sister Chromatid Exchange".Mol Cell Biol. 2017 May 16;37(11):e00161-17. doi: 10.1128/MCB.00161-17. Print 2017 Jun 1. Mol Cell Biol. 2017. PMID: 28512214 Free PMC article. No abstract available.
Abstract
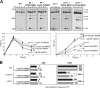
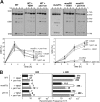
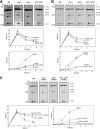
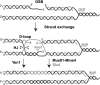
Most spontaneous DNA double-strand breaks (DSBs) arise during replication and are repaired by homologous recombination (HR) with the sister chromatid. Many proteins participate in HR, but it is often difficult to determine their in vivo functions due to the existence of alternative pathways. Here we take advantage of an in vivo assay to assess repair of a specific replication-born DSB by sister chromatid recombination (SCR). We analyzed the functional relevance of four structure-selective endonucleases (SSEs), Yen1, Mus81-Mms4, Slx1-Slx4, and Rad1, on SCR in Saccharomyces cerevisiae. Physical and genetic analyses showed that ablation of any of these SSEs leads to a specific SCR decrease that is not observed in general HR. Our work suggests that Yen1, Mus81-Mms4, Slx4, and Rad1, but not Slx1, function independently in the cleavage of intercrossed DNA structures to reconstitute broken replication forks via HR with the sister chromatid. These unique effects, which have not been detected in other studies unless double mutant combinations were used, indicate the formation of distinct alternatives for the repair of replication-born DSBs that require specific SSEs.
Figures




Similar articles
-
Functional overlap between the structure-specific nucleases Yen1 and Mus81-Mms4 for DNA-damage repair in S. cerevisiae.DNA Repair (Amst). 2010 Apr 4;9(4):394-402. doi: 10.1016/j.dnarep.2009.12.017. Epub 2010 Jan 27. DNA Repair (Amst). 2010. PMID: 20106725
-
Mph1 and Mus81-Mms4 prevent aberrant processing of mitotic recombination intermediates.Mol Cell. 2013 Oct 10;52(1):63-74. doi: 10.1016/j.molcel.2013.09.007. Mol Cell. 2013. PMID: 24119400 Free PMC article.
-
Subnuclear Relocalization of Structure-Specific Endonucleases in Response to DNA Damage.Cell Rep. 2017 Aug 15;20(7):1553-1562. doi: 10.1016/j.celrep.2017.07.059. Cell Rep. 2017. PMID: 28813668
-
Resolving branched DNA intermediates with structure-specific nucleases during replication in eukaryotes.Chromosoma. 2013 Dec;122(6):499-515. doi: 10.1007/s00412-013-0431-z. Epub 2013 Sep 6. Chromosoma. 2013. PMID: 24008669 Free PMC article. Review.
-
Exploring the roles of Mus81-Eme1/Mms4 at perturbed replication forks.DNA Repair (Amst). 2007 Jul 1;6(7):1004-17. doi: 10.1016/j.dnarep.2007.02.019. Epub 2007 Apr 3. DNA Repair (Amst). 2007. PMID: 17409028 Review.
Cited by
-
Break-induced replication and genome stability.Biomolecules. 2012 Dec 1;2(4):483-504. doi: 10.3390/biom2040483. Biomolecules. 2012. PMID: 23767011 Free PMC article.
-
Resolvases, Dissolvases, and Helicases in Homologous Recombination: Clearing the Road for Chromosome Segregation.Genes (Basel). 2020 Jan 8;11(1):71. doi: 10.3390/genes11010071. Genes (Basel). 2020. PMID: 31936378 Free PMC article. Review.
-
Premature Cdk1/Cdc5/Mus81 pathway activation induces aberrant replication and deleterious crossover.EMBO J. 2013 Apr 17;32(8):1155-67. doi: 10.1038/emboj.2013.67. Epub 2013 Mar 26. EMBO J. 2013. PMID: 23531881 Free PMC article.
-
Mus81-Mms4 functions as a single heterodimer to cleave nicked intermediates in recombinational DNA repair.Mol Cell Biol. 2012 Aug;32(15):3065-80. doi: 10.1128/MCB.00547-12. Epub 2012 May 29. Mol Cell Biol. 2012. PMID: 22645308 Free PMC article.
-
A mutation in the endonuclease domain of mouse MLH3 reveals novel roles for MutLγ during crossover formation in meiotic prophase I.PLoS Genet. 2019 Jun 6;15(6):e1008177. doi: 10.1371/journal.pgen.1008177. eCollection 2019 Jun. PLoS Genet. 2019. PMID: 31170160 Free PMC article.
References
-
- Aguilera A, Gomez-Gonzalez B. 2008. Genome instability: a mechanistic view of its causes and consequences. Nat. Rev. Genet. 9:204–217 - PubMed
-
- Blanco MG, Matos J, Rass U, Ip SC, West SC. 2010. Functional overlap between the structure-specific nucleases Yen1 and Mus81-Mms4 for DNA-damage repair in S. cerevisiae. DNA Repair (Amst.). 9:394–402 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
