Polar localization of a symbiosis-specific phosphate transporter is mediated by a transient reorientation of secretion
- PMID: 22355114
- PMCID: PMC3306687
- DOI: 10.1073/pnas.1110215109
Polar localization of a symbiosis-specific phosphate transporter is mediated by a transient reorientation of secretion
Abstract
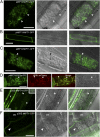
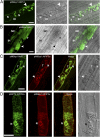
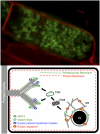
The arbuscular mycorrhizal (AM) symbiosis, formed by land plants and AM fungi, evolved an estimated 400 million years ago and has been maintained in angiosperms, gymnosperms, pteridophytes, and some bryophytes as a strategy for enhancing phosphate acquisition. During AM symbiosis, the AM fungus colonizes the root cortical cells where it forms branched hyphae called arbuscules that function in nutrient exchange with the plant. Each arbuscule is enveloped in a plant membrane, the periarbuscular membrane, that contains a unique set of proteins including phosphate transporters such as Medicago truncatula MtPT4 [Javot et al., (2007) Proc Natl Acad Sci USA 104:1720-1725], which are essential for symbiotic phosphate transport. The periarbuscular membrane is physically continuous with the plasma membrane of the cortical cell, but MtPT4 and other periarbuscular membrane-resident proteins are located only in the domain around the arbuscule branches. Establishing the distinct protein composition of the periarbuscular membrane is critical for AM symbiosis, but currently the mechanism by which this composition is achieved is unknown. Here we investigate the targeting of MtPT4 to the periarbuscular membrane. By expressing MtPT4 and other plasma membrane proteins from promoters active at different phases of the symbiosis, we show that polar targeting of MtPT4 is mediated by precise temporal expression coupled with a transient reorientation of secretion and alterations in the protein cargo entering the secretory system of the colonized root cell. In addition, analysis of phosphate transporter mutants implicates the trans-Golgi network in phosphate transporter secretion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Medicago truncatula mtpt4 mutants reveal a role for nitrogen in the regulation of arbuscule degeneration in arbuscular mycorrhizal symbiosis.Plant J. 2011 Dec;68(6):954-65. doi: 10.1111/j.1365-313X.2011.04746.x. Epub 2011 Oct 17. Plant J. 2011. PMID: 21848683
-
Live-cell imaging reveals periarbuscular membrane domains and organelle location in Medicago truncatula roots during arbuscular mycorrhizal symbiosis.Plant Physiol. 2009 Oct;151(2):809-19. doi: 10.1104/pp.109.141879. Epub 2009 Aug 19. Plant Physiol. 2009. PMID: 19692536 Free PMC article.
-
A set of fluorescent protein-based markers expressed from constitutive and arbuscular mycorrhiza-inducible promoters to label organelles, membranes and cytoskeletal elements in Medicago truncatula.Plant J. 2014 Dec;80(6):1151-63. doi: 10.1111/tpj.12706. Plant J. 2014. PMID: 25329881
-
Arbuscular mycorrhizal symbiosis and plant aquaporin expression.Phytochemistry. 2007 Jan;68(1):122-9. doi: 10.1016/j.phytochem.2006.09.033. Epub 2006 Nov 15. Phytochemistry. 2007. PMID: 17109903 Review.
-
Cellular programs for arbuscular mycorrhizal symbiosis.Curr Opin Plant Biol. 2012 Dec;15(6):691-8. doi: 10.1016/j.pbi.2012.08.010. Epub 2012 Oct 1. Curr Opin Plant Biol. 2012. PMID: 23036821 Review.
Cited by
-
Cellular mechanisms for cargo delivery and polarity maintenance at different polar domains in plant cells.Cell Discov. 2016 Jul 19;2:16018. doi: 10.1038/celldisc.2016.18. eCollection 2016. Cell Discov. 2016. PMID: 27462465 Free PMC article.
-
Two putative-aquaporin genes are differentially expressed during arbuscular mycorrhizal symbiosis in Lotus japonicus.BMC Plant Biol. 2012 Oct 9;12:186. doi: 10.1186/1471-2229-12-186. BMC Plant Biol. 2012. PMID: 23046713 Free PMC article.
-
Cross-kingdom nutrient exchange in the plant-arbuscular mycorrhizal fungus-bacterium continuum.Nat Rev Microbiol. 2024 Dec;22(12):773-790. doi: 10.1038/s41579-024-01073-7. Epub 2024 Jul 16. Nat Rev Microbiol. 2024. PMID: 39014094 Review.
-
RAM1 and RAM2 function and expression during arbuscular mycorrhizal symbiosis and Aphanomyces euteiches colonization.Plant Signal Behav. 2013 Oct;8(10):e26049. doi: 10.4161/psb.26049. Plant Signal Behav. 2013. PMID: 24270627 Free PMC article.
-
Selenium Biofortification of Crop Food by Beneficial Microorganisms.J Fungi (Basel). 2020 May 3;6(2):59. doi: 10.3390/jof6020059. J Fungi (Basel). 2020. PMID: 32375266 Free PMC article. Review.
References
-
- Parniske M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat Rev Microbiol. 2008;6:763–775. - PubMed
-
- Bonfante P, Genre A. (2010) Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nature Communications, 10.1038/ncomms1046. - PubMed
-
- van der Heijden MGA, et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature. 1998;396:69–72.
-
- Alexander T, Toth R, Meier R, Weber HC. Dynamics of arbuscule development and degeneration in onion, bean and tomato with reference to vesicular-arbuscular mycorrhizae in grasses. Can J Bot. 1989;67:2505–2513.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

