Pathogenesis and transmission of swine origin A(H3N2)v influenza viruses in ferrets
- PMID: 22355116
- PMCID: PMC3309732
- DOI: 10.1073/pnas.1119945109
Pathogenesis and transmission of swine origin A(H3N2)v influenza viruses in ferrets
Abstract
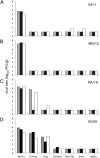
Recent isolation of a novel swine-origin influenza A H3N2 variant virus [A(H3N2)v] from humans in the United States has raised concern over the pandemic potential of these viruses. Here, we analyzed the virulence, transmissibility, and receptor-binding preference of four A(H3N2)v influenza viruses isolated from humans in 2009, 2010, and 2011. High titers of infectious virus were detected in nasal turbinates and nasal wash samples of A(H3N2)v-inoculated ferrets. All four A(H3N2)v viruses possessed the capacity to spread efficiently between cohoused ferrets, and the 2010 and 2011 A(H3N2)v isolates transmitted efficiently to naïve ferrets by respiratory droplets. A dose-dependent glycan array analysis of A(H3N2)v showed a predominant binding to α2-6-sialylated glycans, similar to human-adapted influenza A viruses. We further tested the viral replication efficiency of A(H3N2)v viruses in a relevant cell line, Calu-3, derived from human bronchial epithelium. The A(H3N2)v viruses replicated in Calu-3 cells to significantly higher titers compared with five common seasonal H3N2 influenza viruses. These findings suggest that A(H3N2)v viruses have the capacity for efficient replication and transmission in mammals and underscore the need for continued public health surveillance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Barker WH, Mullooly JP. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol. 1980;112:798–811. - PubMed
-
- Thompson WW, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. - PubMed
-
- Dawood FS, et al. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. - PubMed
-
- Louie JK, et al. California Pandemic (H1N1) Working Group Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–1902. - PubMed
-
- Perez-Padilla R, et al. INER Working Group on Influenza Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

