Full regeneration of the tribasal Polypterus fin
- PMID: 22355122
- PMCID: PMC3309738
- DOI: 10.1073/pnas.1006619109
Full regeneration of the tribasal Polypterus fin
Abstract
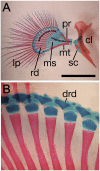
Full limb regeneration is a property that seems to be restricted to urodele amphibians. Here we found that Polypterus, the most basal living ray-finned fish, regenerates its pectoral lobed fins with a remarkable accuracy. Pectoral Polypterus fins are complex, formed by a well-organized endoskeleton to which the exoskeleton rays are connected. Regeneration initiates with the formation of a blastema similar to that observed in regenerating amphibian limbs. Retinoic acid induces dose-dependent phenotypes ranging from inhibition of regeneration to apparent anterior-posterior duplications. As in all developing tetrapod limbs and regenerating amphibian blastema, Sonic hedgehog is expressed in the posterior mesenchyme during fin regeneration. Hedgehog signaling plays a role in the regeneration and patterning processes: an increase or reduction of fin bony elements results when this signaling is activated or disrupted, respectively. The tail fin also regenerates but, in contrast with pectoral fins, regeneration can resume after release from the arrest caused by hedgehog inhibition. A comparative analysis of fin phenotypes obtained after retinoic acid treatment or altering the hedgehog signaling levels during regeneration allowed us to assign a limb tetrapod equivalent segment to Polypterus fin skeletal structures, thus providing clues to the origin of the autopod. We propose that appendage regeneration was a common property of vertebrates during the fin to limb transition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Coates MI. The origin of vertebrate limbs. Dev Suppl. 1994:169–180. - PubMed
-
- Bartsch P, Gemballa S, Piotrowski T. The embryonic and larval development of Polypterus senegalus Cuvier 1829: Its staging by external and skeletal features, behaviour and undulatory locomotion. Acta Zoologica. 1997;78:309–328.
-
- Clack JA. Earliest known tetrapod braincase and the evolution of the stapes and fenestra ovalis. Nature. 1994;369:392–394.
-
- Coates MI, Clack JA. Fish-like gills and breathing in the earliest known tetrapod. Nature. 1991;352:234–236.
-
- Budgett JS. On the structure of the larval Polypterus. T Zool Soc London. 1902;16:315–338.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

