The molten globule state is unusually deformable under mechanical force
- PMID: 22355138
- PMCID: PMC3309780
- DOI: 10.1073/pnas.1115519109
The molten globule state is unusually deformable under mechanical force
Abstract
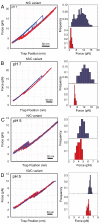
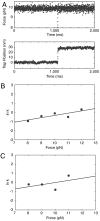
Recently, the role of force in cellular processes has become more evident, and now with advances in force spectroscopy, the response of proteins to force can be directly studied. Such studies have found that native proteins are brittle, and thus not very deformable. Here, we examine the mechanical properties of a class of intermediates referred to as the molten globule state. Using optical trap force spectroscopy, we investigated the response to force of the native and molten globule states of apomyoglobin along different pulling axes. Unlike natively folded proteins, the molten globule state of apomyoglobin is compliant (large distance to the transition state); this large compliance means that the molten globule is more deformable and the unfolding rate is more sensitive to force (the application of force or tension will have a more dramatic effect on the unfolding rate). Our studies suggest that these are general properties of molten globules and could have important implications for mechanical processes in the cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Best RB, Paci E, Hummer G, Dudko OK. Pulling direction as a reaction coordinate for the mechanical unfolding of single molecules. J Phys Chem B. 2008;112:5968–5976. - PubMed
-
- Yew ZT, Schlierf M, Rief M, Paci E. Direct evidence of the multidimensionality of the free-energy landscapes of proteins revealed by mechanical probes. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81:031923. - PubMed
-
- Bustamante C, Chemla YR, Forde NR, Izhaky D. Mechanical processes in biochemistry. Annu Rev Biochem. 2004;73:705–748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

