The distribution of ligand-binding pockets around protein-protein interfaces suggests a general mechanism for pocket formation
- PMID: 22355140
- PMCID: PMC3309739
- DOI: 10.1073/pnas.1117768109
The distribution of ligand-binding pockets around protein-protein interfaces suggests a general mechanism for pocket formation
Abstract
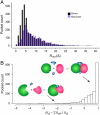
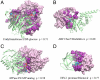
Protein-protein and protein-ligand interactions are ubiquitous in a biological cell. Here, we report a comprehensive study of the distribution of protein-ligand interaction sites, namely ligand-binding pockets, around protein-protein interfaces where protein-protein interactions occur. We inspected a representative set of 1,611 representative protein-protein complexes and identified pockets with a potential for binding small molecule ligands. The majority of these pockets are within a 6 Å distance from protein interfaces. Accordingly, in about half of ligand-bound protein-protein complexes, amino acids from both sides of a protein interface are involved in direct contacts with at least one ligand. Statistically, ligands are closer to a protein-protein interface than a random surface patch of the same solvent accessible surface area. Similar results are obtained in an analysis of the ligand distribution around domain-domain interfaces of 1,416 nonredundant, two-domain protein structures. Furthermore, comparable sized pockets as observed in experimental structures are present in artificially generated protein complexes, suggesting that the prominent appearance of pockets around protein interfaces is mainly a structural consequence of protein packing and thus, is an intrinsic geometric feature of protein structure. Nature may take advantage of such a structural feature by selecting and further optimizing for biological function. We propose that packing nearby protein-protein or domain-domain interfaces is a major route to the formation of ligand-binding pockets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


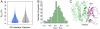
References
-
- Alberts B. Molecular biology of the cell. 5th Ed. New York: Garland Science; 2008.
-
- Janin J, Bahadur RP, Chakrabarti P. Protein-protein interaction and quaternary structure. Q Rev Biophys. 2008;41:133–180. - PubMed
-
- Keskin Z, Gursoy A, Ma B, Nussinov R. Principles of protein-protein interactions: What are the preferred ways for proteins to interact? Chem Rev. 2008;108:1225–1244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

