Evidence for an intermediate conformational state of LacY
- PMID: 22355148
- PMCID: PMC3311394
- DOI: 10.1073/pnas.1201107109
Evidence for an intermediate conformational state of LacY
Abstract
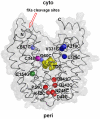
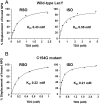
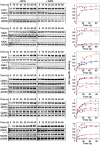
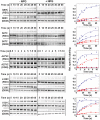
LacY mutant Cys154 → Gly exhibits a periplasmic-closed crystal structure identical to the WT, but is periplasmic-open in the membrane. The mutant hardly catalyzes transport, but binds galactosides from either side of the membrane with the same affinity and is resistant to site-directed proteolysis relative to the pseudo-WT. Site-directed alkylation was also applied to 11 single-Cys mutants in Cys154 → Gly LacY in right-side-out membrane vesicles or after solubilization and purification in dodecyl-β-D-maltopyranoside (DDM). Unlike the pseudo-WT, Cys replacements on the periplasmic side of the Cys154 → Gly mutant label rapidly in the membrane without sugar, but labeling decreases markedly after the mutant proteins are purified. Thus, Cys154 → Gly LacY likely favors a higher-energy intermediate periplasmic-open conformation in situ, but collapses to a lower-energy periplasmic-closed conformation in DDM after purification. Notably, branched-chain or neopentyl glycol maltoside detergents stabilize Cys154 → Gly LacY in the membrane-embedded form.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
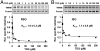

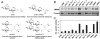


References
-
- Kaback HR, Sahin-Toth M, Weinglass AB. The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol. 2001;2:610–620. - PubMed
-
- Viitanen P, Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. - PubMed
-
- Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

