The genetic control of avascular area in mouse oxygen-induced retinopathy
- PMID: 22355249
- PMCID: PMC3283213
The genetic control of avascular area in mouse oxygen-induced retinopathy
Abstract
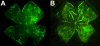
Purpose: The C57BL/6ByJ and BALB/cByJ inbred strains of mice are, respectively, susceptible and resistant to oxygen-induced retinopathy (OIR). The purpose of this work was to investigate the genetic control of the retinal avascular area in mouse OIR using a mapping cross.
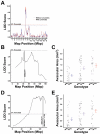
Methods: The central retinal avascular area was measured on postnatal day 16 (P16) in C57BL/6ByJ, BALB/cByJ, 101 (C57BL/6ByJ x BALB/cByJ)F₂, and 116 (BALB/cByJ x C57BL/6ByJ)F₂ mice that had been subjected to the OIR protocol. A genome-wide scan was performed of selected albino and non-albino mice to determine quantitative trait loci associated with weight and avascular area.
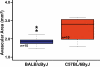
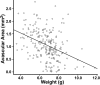
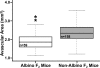
Results: C57BL/6ByJ mice had significantly larger avascular areas than BALB/cByJ ones. Albino mice of the F₂ generation had smaller avascular areas than the non-albino mice. Genotyping was performed at 856 informative single nucleotide polymorphisms approximately evenly distributed across the genome from each of 85 selected F₂ mice. Weight, sex, and the paternal grandmother were found to act as additive covariates associated with the avascular area on P16; mapping analyses that used a model incorporating these covariates found a quantitative trait locus on chromosome 7 related to avascular area. Mapping analyses that used a model that did not incorporate covariates found a quantitative trait locus on chromosome 9 related to avascular area. A quantitative trait locus for bodyweight on P16 was mapped to chromosome 5.
Conclusions: The retinal avascular area in the mouse OIR model is under genetic control. Revascularization in OIR is related to the weight, strain of paternal grandmother, sex, and albinism. Our data support the existence of a quantitative trait locus on chromosome 5 that influences weight after exposure to hyperoxia, as well as quantitative trait loci on chromosomes 7 and 9 that modify susceptibility to OIR.
Figures


References
-
- Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. - PubMed
-
- Bizzarro MJ, Hussain N, Jonsson B, Feng R, Ment LR, Gruen JR, Zhang H, Bhandari V. Genetic susceptibility to retinopathy of prematurity. Pediatrics. 2006;118:1858–63. - PubMed
-
- Kim TI, Sohn J, Pi SY, Yoon YH. Postnatal risk factors of retinopathy of prematurity. Paediatr Perinat Epidemiol. 2004;18:130–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
