Correlation transfer from basal ganglia to thalamus in Parkinson's disease
- PMID: 22355287
- PMCID: PMC3280480
- DOI: 10.3389/fncom.2011.00058
Correlation transfer from basal ganglia to thalamus in Parkinson's disease
Abstract
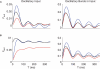
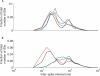
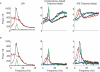
Spike trains from neurons in the basal ganglia of parkinsonian primates show increased pairwise correlations, oscillatory activity, and burst rate compared to those from neurons recorded during normal brain activity. However, it is not known how these changes affect the behavior of downstream thalamic neurons. To understand how patterns of basal ganglia population activity may affect thalamic spike statistics, we study pairs of model thalamocortical (TC) relay neurons receiving correlated inhibitory input from the internal segment of the globus pallidus (GPi), a primary output nucleus of the basal ganglia. We observe that the strength of correlations of TC neuron spike trains increases with the GPi correlation level, and bursty firing patterns such as those seen in the parkinsonian GPi allow for stronger transfer of correlations than do firing patterns found under normal conditions. We also show that the T-current in the TC neurons does not significantly affect correlation transfer, despite its pronounced effects on spiking. Oscillatory firing patterns in GPi are shown to affect the timescale at which correlations are best transferred through the system. To explain this last result, we analytically compute the spike count correlation coefficient for oscillatory cases in a reduced point process model. Our analysis indicates that the dependence of the timescale of correlation transfer is robust to different levels of input spike and rate correlations and arises due to differences in instantaneous spike correlations, even when the long timescale rhythmic modulations of neurons are identical. Overall, these results show that parkinsonian firing patterns in GPi do affect the transfer of correlations to the thalamus.
Keywords: Parkinson's disease; basal ganglia; bursting; correlation; oscillations; point process; synchrony; thalamus.
Figures



Similar articles
-
Healthy and pathological pallidal regulation of thalamic burst versus tonic mode firing: a computational simulation.Neuroreport. 2023 Nov 1;34(16):773-780. doi: 10.1097/WNR.0000000000001955. Epub 2023 Sep 19. Neuroreport. 2023. PMID: 37756165
-
Effects of high-frequency stimulation of the internal pallidal segment on neuronal activity in the thalamus in parkinsonian monkeys.J Neurophysiol. 2016 Dec 1;116(6):2869-2881. doi: 10.1152/jn.00104.2016. Epub 2016 Sep 28. J Neurophysiol. 2016. PMID: 27683881 Free PMC article.
-
The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism.J Neurophysiol. 1994 Aug;72(2):507-20. doi: 10.1152/jn.1994.72.2.507. J Neurophysiol. 1994. PMID: 7983515
-
Pathophysiology of Parkinson's disease: the MPTP primate model of the human disorder.Ann N Y Acad Sci. 2003 Jun;991:199-213. doi: 10.1111/j.1749-6632.2003.tb07477.x. Ann N Y Acad Sci. 2003. PMID: 12846988 Review.
-
Basal ganglia, movement disorders and deep brain stimulation: advances made through non-human primate research.J Neural Transm (Vienna). 2018 Mar;125(3):419-430. doi: 10.1007/s00702-017-1736-5. Epub 2017 Jun 10. J Neural Transm (Vienna). 2018. PMID: 28601961 Free PMC article. Review.
Cited by
-
Reduced information transmission in the internal segment of the globus pallidus of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced rhesus monkey models of Parkinson's disease.Neural Regen Res. 2012 Sep 15;7(26):2028-35. doi: 10.3969/j.issn.1673-5374.2012.26.004. Neural Regen Res. 2012. PMID: 25624834 Free PMC article.
-
Exploring the role of striatal D1 and D2 medium spiny neurons in action selection using a virtual robotic framework.Eur J Neurosci. 2019 Mar;49(6):737-753. doi: 10.1111/ejn.14021. Epub 2018 Aug 1. Eur J Neurosci. 2019. PMID: 29917291 Free PMC article.
-
Using a hybrid neuron in physiologically inspired models of the basal ganglia.Front Comput Neurosci. 2013 Jul 5;7:88. doi: 10.3389/fncom.2013.00088. eCollection 2013. Front Comput Neurosci. 2013. PMID: 23847524 Free PMC article.
-
Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects.Eur J Neurosci. 2012 Jul;36(2):2213-28. doi: 10.1111/j.1460-9568.2012.08108.x. Eur J Neurosci. 2012. PMID: 22805066 Free PMC article. Review.
-
Intrinsic timescales and predictive allostatic interoception in brain health and disease.Neurosci Biobehav Rev. 2024 Feb;157:105510. doi: 10.1016/j.neubiorev.2023.105510. Epub 2023 Dec 15. Neurosci Biobehav Rev. 2024. PMID: 38104789 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

