Antibacterial and antifungal activities of new acylated derivatives of epigallocatechin gallate
- PMID: 22355295
- PMCID: PMC3280433
- DOI: 10.3389/fmicb.2012.00053
Antibacterial and antifungal activities of new acylated derivatives of epigallocatechin gallate
Abstract
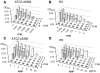
(-)-Epigallocatechin-3-O-gallate (EGCG) has useful antiviral, antimicrobial, antitoxin, and antitumor properties. Previously, Mori et al. (2008) found that addition of long acyl chains (C16-18) to EGCG enhanced its anti-influenza virus activity up to 44-fold. The chemical stability of EGCG against oxidative degradation was also enhanced by acylation. We further evaluated the in vitro activity spectrum of the EGCG derivatives against a wide range of bacteria and fungi. A series of EGCG O-acyl derivatives were synthesized by lipase-catalyzed transesterification. These derivatives exhibited several-fold higher activities than EGCG, particularly against Gram-positive organisms. Antifungal MICs of the derivatives were also two to fourfold lower than those of EGCG. The activities of the EGCG derivatives against Gram-negative bacteria were not distinguishable from those of EGCG. Among the derivatives evaluated, MICs of dioctanoate and palmitate (C16) for 17 Staphylococcus aureus strains were 4-32 μg/ml, although MIC of EGCG for these 17 strains was ≥128 μg/ml. C16 demonstrated rapid bactericidal activity against methicillin-resistant S. aureus (MRSA) ATCC43300 at ≥16 μg/ml. The enhanced activity of C16 against S. aureus was supported by its increased membrane-permeabilizing activity determined by increased SYTOX Green uptake. The EGCG derivatives were exported in Escherichia coli using the efflux pump AcrAB-TolC. The tolC deletion mutant exhibited higher sensitivity to EGCG and the derivatives than wild-type. Addition of long alkyl chains to EGCG significantly enhanced its activities against several bacteria and fungi, particularly against S. aureus including MRSA. C16 might potentially become under specified circumstances an alternative or supplement to antibiotics and disinfectants in the future.
Keywords: bactericidal activity Staphylococcus aureus; epigallocatechin gallate; fatty acid esters.
Figures
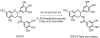

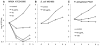

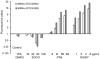
Similar articles
-
Enhanced antitumor activities of (-)-epigallocatechin-3-O-gallate fatty acid monoester derivatives in vitro and in vivo.Biochem Biophys Res Commun. 2008 Dec 26;377(4):1118-22. doi: 10.1016/j.bbrc.2008.10.128. Epub 2008 Nov 5. Biochem Biophys Res Commun. 2008. PMID: 18983978
-
Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea.Br J Pharmacol. 2013 Mar;168(5):1059-73. doi: 10.1111/bph.12009. Br J Pharmacol. 2013. PMID: 23072320 Free PMC article. Review.
-
Antibacterial activity of epigallocatechin gallate against methicillin-resistant Staphylococcus aureus.Kansenshogaku Zasshi. 1994 Dec;68(12):1518-22. doi: 10.11150/kansenshogakuzasshi1970.68.1518. Kansenshogaku Zasshi. 1994. PMID: 7876674
-
Co-assembled nanocomplexes comprising epigallocatechin gallate and berberine for enhanced antibacterial activity against multidrug resistant Staphylococcus aureus.Biomed Pharmacother. 2023 Jul;163:114856. doi: 10.1016/j.biopha.2023.114856. Epub 2023 May 16. Biomed Pharmacother. 2023. PMID: 37196539
-
In-vitro profile of a new beta-lactam, ceftobiprole, with activity against methicillin-resistant Staphylococcus aureus.Clin Microbiol Infect. 2007 Jun;13 Suppl 2:17-24. doi: 10.1111/j.1469-0691.2007.01722.x. Clin Microbiol Infect. 2007. PMID: 17488372 Review.
Cited by
-
Regular Consumption of Green Tea as an Element of Diet Therapy in Drug-Induced Liver Injury (DILI).Nutrients. 2024 Aug 24;16(17):2837. doi: 10.3390/nu16172837. Nutrients. 2024. PMID: 39275155 Free PMC article. Review.
-
Differential Hydraulic Properties and Primary Metabolism in Fine Root of Avocado Trees Rootstocks.Plants (Basel). 2022 Apr 13;11(8):1059. doi: 10.3390/plants11081059. Plants (Basel). 2022. PMID: 35448786 Free PMC article.
-
Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties.Biomed Res Int. 2015;2015:905215. doi: 10.1155/2015/905215. Epub 2015 Feb 23. Biomed Res Int. 2015. PMID: 25802870 Free PMC article. Review.
-
Beneficial Effects of Green Tea EGCG on Skin Wound Healing: A Comprehensive Review.Molecules. 2021 Oct 11;26(20):6123. doi: 10.3390/molecules26206123. Molecules. 2021. PMID: 34684703 Free PMC article. Review.
-
In silico prediction of flavan-3-ol as a bioactive compound of Calophyllum macrophyllum as a potential drug against angiostrongylus eosinophilic meningitis.Vet World. 2022 May;15(5):1305-1313. doi: 10.14202/vetworld.2022.1305-1313. Epub 2022 May 25. Vet World. 2022. PMID: 35765470 Free PMC article.
References
-
- Clinical and Laboratory Standards Institute (2009). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Eighth Edition. Wayne: Clinical and Laboratory Standards Institute
-
- Das D. N. (1962). Studies on the antibiotic activity of tea. J. Indian Chem. Soc. 33, 849–854
-
- Davis R. W., Botstein D., Roth J. R. (1980). Advanced Bacterial Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory
-
- Green R. H. (1949). Inhibition of multiplication of influenza virus by extracts of tea. Proc. Soc. Exp. Biol. Med. 71, 84. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

