Nanobiopolymer for direct targeting and inhibition of EGFR expression in triple negative breast cancer
- PMID: 22355336
- PMCID: PMC3280290
- DOI: 10.1371/journal.pone.0031070
Nanobiopolymer for direct targeting and inhibition of EGFR expression in triple negative breast cancer
Abstract
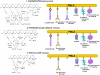
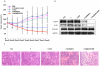
Treatment options for triple negative breast cancer (TNBC) are generally limited to cytotoxic chemotherapy. Recently, anti-epidermal growth factor receptor (EGFR) therapy has been introduced for TNBC patients. We engineered a novel nanobioconjugate based on a poly(β-L-malic acid) (PMLA) nanoplatform for TNBC treatment. The nanobioconjugate carries anti-tumor nucleosome-specific monoclonal antibody (mAb) 2C5 to target breast cancer cells, anti-mouse transferrin receptor (TfR) antibody for drug delivery through the host endothelial system, and Morpholino antisense oligonucleotide (AON) to inhibit EGFR synthesis. The nanobioconjugates variants were: (1) P (BioPolymer) with AON, 2C5 and anti-TfR for tumor endothelial and cancer cell targeting, and EGFR suppression (P/AON/2C5/TfR), and (2) P with AON and 2C5 (P/AON/2C5). Controls included (3) P with 2C5 but without AON (P/2C5), (4) PBS, and (5) P with PEG and leucine ester (LOEt) for endosomal escape (P/mPEG/LOEt). Drugs were injected intravenously to MDA-MB-468 TNBC bearing mice. Tissue accumulation of injected nanobioconjugates labeled with Alexa Fluor 680 was examined by Xenogen IVIS 200 (live imaging) and confocal microscopy of tissue sections. Levels of EGFR, phosphorylated and total Akt in tumor samples were detected by western blotting. In vitro western blot showed that the leading nanobioconjugate P/AON/2C5/TfR inhibited EGFR synthesis significantly better than naked AON. In vivo imaging revealed that 2C5 increased drug-tumor accumulation. Significant tumor growth inhibition was observed in mice treated with the lead nanobioconjugate (1) [P = 0.03 vs. controls; P<0.05 vs. nanobioconjugate variant (2)]. Lead nanobioconjugate (1) also showed stronger inhibition of EGFR expression and Akt phosphorylation than other treatments. Treatment of TNBC with the new nanobioconjugate results in tumor growth arrest by inhibiting EGFR and its downstream signaling intermediate, phosphorylated Akt. The nanobioconjugate represents a new generation of nanodrugs for treatment of TNBC.
Conflict of interest statement
Figures



References
-
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. - PubMed
-
- Bevers TB, Anderson BO, Bonaccio E, Buys S, Daly MB, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7:1060–1096. - PubMed
-
- Agrawal A, Gutteridge E, Gee JM, Nicholson RI, Robertson JF. Overview of tyrosine kinase inhibitors in clinical breast cancer. Endocr Relat Cancer. 2005;12(Suppl 1):S135–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

