TGFbeta family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages
- PMID: 22355352
- PMCID: PMC3280291
- DOI: 10.1371/journal.pone.0031274
TGFbeta family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages
Abstract
Objective: The present study was undertaken to characterize the remodeling phenotype of human adipose tissue (AT) macrophages (ATM) and to analyze their paracrine effects on AT progenitor cells.
Research design and methods: The phenotype of ATM, immunoselected from subcutaneous (Sc) AT originating from subjects with wide range of body mass index and from paired biopsies of Sc and omental (Om) AT from obese subjects, was studied by gene expression analysis in the native and activated states. The paracrine effects of ScATM on the phenotype of human ScAT progenitor cells (CD34(+)CD31(-)) were investigated.
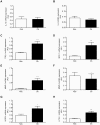
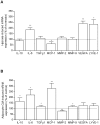
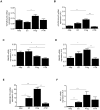
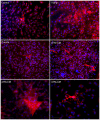
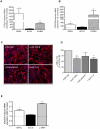
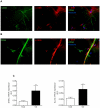
Results: Two main ATM phenotypes were distinguished based on gene expression profiles. For ScAT-derived ATM, obesity and adipocyte-derived factors favored a pro-fibrotic/remodeling phenotype whereas the OmAT location and hypoxic culture conditions favored a pro-angiogenic phenotype. Treatment of native human ScAT progenitor cells with ScATM-conditioned media induced the appearance of myofibroblast-like cells as shown by expression of both α-SMA and the transcription factor SNAIL, an effect mimicked by TGFβ1 and activinA. Immunohistochemical analyses showed the presence of double positive α-SMA and CD34 cells in the stroma of human ScAT. Moreover, the mRNA levels of SNAIL and SLUG in ScAT progenitor cells were higher in obese compared with lean subjects.
Conclusions: Human ATM exhibit distinct pro-angiogenic and matrix remodeling/fibrotic phenotypes according to the adiposity and the location of AT, that may be related to AT microenvironment including hypoxia and adipokines. Moreover, human ScAT progenitor cells have been identified as target cells for ScATM-derived TGFβ and as a potential source of fibrosis through their induction of myofibroblast-like cells.
Conflict of interest statement
Figures

References
-
- Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. - PubMed
-
- Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

