Osteoadherin accumulates in the predentin towards the mineralization front in the developing tooth
- PMID: 22355375
- PMCID: PMC3280325
- DOI: 10.1371/journal.pone.0031525
Osteoadherin accumulates in the predentin towards the mineralization front in the developing tooth
Abstract
Background: Proteoglycans (PG) are known to be involved in the organization and assembly of the extracellular matrix (ECM) prior to mineral deposition. Osteoadherin (OSAD), a keratan sulphate PG is a member of the small leucine-rich (SLRP) family of PGs and unlike other SLRPs, OSAD expression is restricted to mineralized tissues. It is proposed to have a high affinity for hydroxyapatite and has been shown to be expressed by mature osteoblasts but its exact role remains to be elucidated.
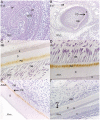
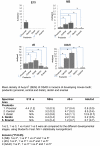
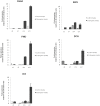
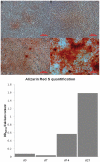
Methodology/principal findings: We investigated the protein distribution of OSAD in the developing mouse tooth using immunohistochemistry and compared its expression with other SLRPs, biglycan (BGN), decorin (DCN) and fibromodulin (FMD). OSAD was found to be specifically localized in the predentin layer of the tooth and focused at the mineralization front. These studies were confirmed at the ultrastructural level using electron microscopy (iEM), where the distribution of immunogold labeled OSAD particles were quantified and significant amounts were found in the predentin, forming a gradient towards the mineralization front. In addition, iEM results revealed OSAD to lie in close association with collagen fibers, further suggesting an important role for OSAD in the organization of the ECM. The expression profile of mineralization-related SLRP genes by rat dental pulp cells exposed to mineralization inducing factors, showed an increase in all SLRP genes. Indeed, OSAD expression was significantly increased during the mineralization process, specifically following, matrix maturation, and finally mineral deposition. Alizarin Red S staining for calcium deposition showed clear bone-like nodules, which support matrix maturation and mineralization.
Conclusions: These studies provide new evidence for the role of OSAD in the mineralization process and its specific localization in the predentin layer accumulating at the mineralization front highlighting its role in tooth development.
Conflict of interest statement
Figures


References
-
- Goldberg M, Septier D, Lecolle S, Chardin H, Quintana MA, et al. Dental mineralization. Int J Dev Biol. 1995;39:93–110. - PubMed
-
- Zhou HY. Proteomic analysis of hydroxyapatite interaction proteins in bone. Ann N Y Acad Sci. 2007;1116:323–326. - PubMed
-
- Embery G, Hall R, Waddington R, Septier D, Goldberg M. Proteoglycans in dentinogenesis. Crit Rev Oral Biol Med. 2001;12:331–349. - PubMed
-
- Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17:1–19. - PubMed
-
- Goldberg M, Takagi M. Dentine proteoglycans: composition, ultrastructure and functions. Histochem J. 1993;25:781–806. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

