The human antibody response to dengue virus infection
- PMID: 22355444
- PMCID: PMC3280510
- DOI: 10.3390/v3122374
The human antibody response to dengue virus infection
Abstract
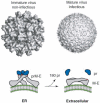
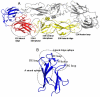
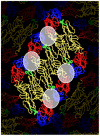
Dengue viruses (DENV) are the causative agents of dengue fever (DF) and dengue hemorrhagic fever (DHF). Here we review the current state of knowledge about the human antibody response to dengue and identify important knowledge gaps. A large body of work has demonstrated that antibodies can neutralize or enhance DENV infection. Investigators have mainly used mouse monoclonal antibodies (MAbs) to study interactions between DENV and antibodies. These studies indicate that antibody neutralization of DENVs is a "multi-hit" phenomenon that requires the binding of multiple antibodies to neutralize a virion. The most potently neutralizing mouse MAbs bind to surface exposed epitopes on domain III of the dengue envelope (E) protein. One challenge facing the dengue field now is to extend these studies with mouse MAbs to better understand the human antibody response. The human antibody response is complex as it involves a polyclonal response to primary and secondary infections with 4 different DENV serotypes. Here we review studies conducted with immune sera and MAbs isolated from people exposed to dengue infections. Most dengue-specific antibodies in human immune sera are weakly neutralizing and bind to multiple DENV serotypes. The human antibodies that potently and type specifically neutralize DENV represent a small fraction of the total DENV-specific antibody response. Moreover, these neutralizing antibodies appear to bind to novel epitopes including complex, quaternary epitopes that are only preserved on the intact virion. These studies establish that human and mouse antibodies recognize distinct epitopes on the dengue virion. The leading theory proposed to explain the increased risk of severe disease in secondary cases is antibody dependent enhancement (ADE), which postulates that weakly neutralizing antibodies from the first infection bind to the second serotype and enhance infection of FcγR bearing myeloid cells such as monocytes and macrophages. Here we review results from human, animal and cell culture studies relevant to the ADE hypothesis. By understanding how human antibodies neutralize or enhance DENV, it will be possible to better evaluate existing vaccines and develop the next generation of novel vaccines.
Keywords: antibody; antibody dependent enhancement; dengue virus; neutralization.
Figures

Similar articles
-
Mapping the Human Memory B Cell and Serum Neutralizing Antibody Responses to Dengue Virus Serotype 4 Infection and Vaccination.J Virol. 2017 Feb 14;91(5):e02041-16. doi: 10.1128/JVI.02041-16. Print 2017 Mar 1. J Virol. 2017. PMID: 28031369 Free PMC article.
-
Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions.Proc Natl Acad Sci U S A. 2012 May 8;109(19):7439-44. doi: 10.1073/pnas.1200566109. Epub 2012 Apr 12. Proc Natl Acad Sci U S A. 2012. PMID: 22499787 Free PMC article.
-
Isolation of dengue virus-specific memory B cells with live virus antigen from human subjects following natural infection reveals the presence of diverse novel functional groups of antibody clones.J Virol. 2014 Nov;88(21):12233-41. doi: 10.1128/JVI.00247-14. Epub 2014 Aug 6. J Virol. 2014. PMID: 25100837 Free PMC article.
-
Paradoxical role of antibodies in dengue virus infections: considerations for prophylactic vaccine development.Expert Rev Vaccines. 2016;15(4):467-82. doi: 10.1586/14760584.2016.1121814. Epub 2015 Dec 15. Expert Rev Vaccines. 2016. PMID: 26577689 Review.
-
Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection.Front Immunol. 2018 Apr 23;9:597. doi: 10.3389/fimmu.2018.00597. eCollection 2018. Front Immunol. 2018. PMID: 29740424 Free PMC article. Review.
Cited by
-
A chimeric dengue virus vaccine candidate delivered by high density microarray patches protects against infection in mice.NPJ Vaccines. 2021 May 7;6(1):66. doi: 10.1038/s41541-021-00328-1. NPJ Vaccines. 2021. PMID: 33963191 Free PMC article.
-
Dengue Virus and Vaccines: How Can DNA Immunization Contribute to This Challenge?Front Med Technol. 2021 Apr 12;3:640964. doi: 10.3389/fmedt.2021.640964. eCollection 2021. Front Med Technol. 2021. PMID: 35047911 Free PMC article. Review.
-
Development and Characterization of a Multiplex Assay to Quantify Complement-Fixing Antibodies against Dengue Virus.Int J Mol Sci. 2021 Nov 5;22(21):12004. doi: 10.3390/ijms222112004. Int J Mol Sci. 2021. PMID: 34769432 Free PMC article.
-
Pathogen-specific deep sequence-coupled biopanning: A method for surveying human antibody responses.PLoS One. 2017 Feb 2;12(2):e0171511. doi: 10.1371/journal.pone.0171511. eCollection 2017. PLoS One. 2017. PMID: 28152075 Free PMC article.
-
Development and validation of an assay for detection of Japanese encephalitis virus specific antibody responses.PLoS One. 2020 Oct 28;15(10):e0238609. doi: 10.1371/journal.pone.0238609. eCollection 2020. PLoS One. 2020. PMID: 33112881 Free PMC article.
References
-
- Mackenzie J.S., Gubler D.J., Petersen L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004;10:S98–S109. - PubMed
-
- Roehrig J.T. Antigenic structure of flavivirus proteins. Adv. Virus Res. 2003;59:141–175. - PubMed
-
- Rothman A.L. Immunology and immunopathogenesis of dengue disease. Adv. Virus Res. 2003;60:397–419. - PubMed
-
- Halstead S.B. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 2003;60:421–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

