The curious case of arenavirus entry, and its inhibition
- PMID: 22355453
- PMCID: PMC3280523
- DOI: 10.3390/v4010083
The curious case of arenavirus entry, and its inhibition
Abstract
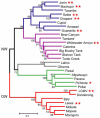
Arenaviruses comprise a diverse family of enveloped negative-strand RNA viruses that are endemic to specific rodent hosts worldwide. Several arenaviruses cause severe hemorrhagic fevers in humans, including Junín and Machupo viruses in South America and Lassa fever virus in western Africa. Arenavirus entry into the host cell is mediated by the envelope glycoprotein complex, GPC. The virion is endocytosed on binding to a cell-surface receptor, and membrane fusion is initiated in response to physiological acidification of the endosome. As with other class I virus fusion proteins, GPC-mediated membrane fusion is promoted through a regulated sequence of conformational changes leading to formation of the classical postfusion trimer-of-hairpins structure. GPC is, however, unique among the class I fusion proteins in that the mature complex retains a stable signal peptide (SSP) as a third subunit, in addition to the canonical receptor-binding and fusion proteins. We will review the curious properties of the tripartite GPC complex and describe evidence that SSP interacts with the fusion subunit to modulate pH-induced activation of membrane fusion. This unusual solution to maintaining the metastable prefusion state of GPC on the virion and activating the class I fusion cascade at acidic pH provides novel targets for antiviral intervention.
Keywords: antiviral; arenavirus; endosome; envelope glycoprotein; fusion inhibitor; fusion protein; hemorrhagic fever; pH-dependent membrane fusion; stable signal peptide; zinc binding.
Figures


References
-
- Charrel R.N., de Lamballerie X. Zoonotic aspects of arenavirus infections. Vet. Microbiol. 2010;140:213–220. - PubMed
-
- Coulibaly-N'Golo D., Allali B., Kouassi S.K., Fichet-Calvet E., Becker-Ziaja B., Rieger T., Olschläger S., Dosso H., Denys C., Ter Meulen J., et al. Novel arenavirus sequences in Hylomyscus sp. and Mus (Nannomys) setulosus from Côte d'Ivoire: Implications for evolution of arenaviruses in Africa. PLoS One. 2011;6:e20893. - PMC - PubMed
-
- Clegg J.C.S. Molecular phylogeny of the arenaviruses. Curr. Top. Microbiol. Immunol. 2002;262:1–24. - PubMed
-
- Salazar-Bravo J., Ruedas L.A., Yates T.L. Mammalian reservoirs of arenaviruses. Curr. Top. Microbiol. Immunol. 2002;262:25–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

