Molecular and cellular aspects of rhabdovirus entry
- PMID: 22355455
- PMCID: PMC3280520
- DOI: 10.3390/v4010117
Molecular and cellular aspects of rhabdovirus entry
Abstract
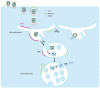
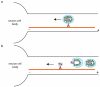
Rhabdoviruses enter the cell via the endocytic pathway and subsequently fuse with a cellular membrane within the acidic environment of the endosome. Both receptor recognition and membrane fusion are mediated by a single transmembrane viral glycoprotein (G). Fusion is triggered via a low-pH induced structural rearrangement. G is an atypical fusion protein as there is a pH-dependent equilibrium between its pre- and post-fusion conformations. The elucidation of the atomic structures of these two conformations for the vesicular stomatitis virus (VSV) G has revealed that it is different from the previously characterized class I and class II fusion proteins. In this review, the pre- and post-fusion VSV G structures are presented in detail demonstrating that G combines the features of the class I and class II fusion proteins. In addition to these similarities, these G structures also reveal some particularities that expand our understanding of the working of fusion machineries. Combined with data from recent studies that revealed the cellular aspects of the initial stages of rhabdovirus infection, all these data give an integrated view of the entry pathway of rhabdoviruses into their host cell.
Keywords: endocytosis; glycoprotein; membrane fusion; rabies virus; rhabdovirus; vesicular stomatitis virus.
Figures
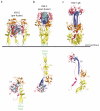
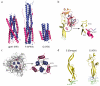
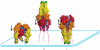
References
-
- Rose J.K., Whitt M.A. Fields Virology. 4th. Lippincott-Raven Publishers; Philadelphia, PA, USA: 2001. Rhabdoviridae: The viruses and their replication; pp. 1221–1224.
-
- Fort P., Albertini A., Van-Hua A., Berthomieu A., Roche S., Delsuc F., Pasteur N., Capy P., Gaudin Y., Weill M. Fossil rhabdoviral sequences integrated into arthropod genomes: Ontogeny, evolution, and potential functionality. Mol. Biol. Evol. 2011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

