Zfp488 promotes oligodendrocyte differentiation of neural progenitor cells in adult mice after demyelination
- PMID: 22355521
- PMCID: PMC3210692
- DOI: 10.1038/srep00002
Zfp488 promotes oligodendrocyte differentiation of neural progenitor cells in adult mice after demyelination
Abstract
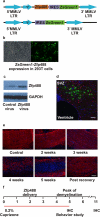
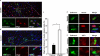
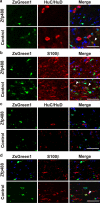
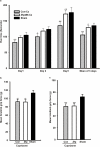
Basic helix-loop-helix transcription factors Olig1 and Olig2 critically regulate oligodendrocyte development. Initially identified as a downstream effector of Olig1, an oligodendrocyte-specific zinc finger transcription repressor, Zfp488, cooperates with Olig2 function. Although Zfp488 is required for oligodendrocyte precursor formation and differentiation during embryonic development, its role in oligodendrogenesis of adult neural progenitor cells is not known. In this study, we tested whether Zfp488 could promote an oligodendrogenic fate in adult subventricular zone (SVZ) neural stem/progenitor cells (NSPCs). Using a cuprizone-induced demyelination model in mice, we examined the effect of retrovirus-mediated Zfp488 overexpression in SVZ NSPCs. Our results showed that Zfp488 efficiently promoted the differentiation of the SVZ NSPCs into mature oligodendrocytes in vivo. After cuprizone-induced demyelination injury, Zfp488-transduced mice also showed significant restoration of motor function to levels comparable to control mice. Together, these findings identify a previously unreported role for Zfp488 in adult oligodendrogenesis and functional remyelination after injury.
Figures

References
-
- Ben-Hur T. & Goldman S.A. Prospects of cell therapy for disorders of myelin. Ann N Y Acad Sci 1142, 218–249 (2008). - PubMed
-
- Franklin R.J. & Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9, 839–855 (2008). - PubMed
-
- Nishiyama A., Lin X.H., Giese N., Heldin C.H. & Stallcup W.B. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res 43, 299–314 (1996). - PubMed
-
- Reynolds R. & Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res 47, 455–470 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

