Modulation of epithelial immunity by mucosal fluid
- PMID: 22355527
- PMCID: PMC3216496
- DOI: 10.1038/srep00008
Modulation of epithelial immunity by mucosal fluid
Abstract
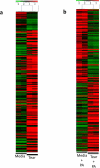
Mucosal epithelial cells, including those at the ocular surface, resist infection by most microbes in vivo but can be susceptible to microbial virulence in vitro. While fluids bathing mucosal surfaces (e.g. tears) contain antimicrobials, potentially pathogenic microbes often thrive in these fluids, suggesting that additional mechanisms mediate epithelial resistance in vivo. Here, tear fluid acted directly upon epithelial cells to enhance their resistance to bacterial invasion and cytotoxicity. Resistance correlated with tear fluid-magnified activation of NFκB and AP-1 transcription factors in epithelial cells in response to bacterial antigens, suggesting priming of innate defense pathways. Further analysis revealed differential regulation of potential epithelial cell defense genes by tears. siRNA knockdown confirmed involvement of at least two factors, RNase7 and ST-2, for which tears increased mRNA levels, in protection against bacterial invasion. Thus, the role of mucosal fluids in defense can include modulation of epithelial immunity, in addition to direct effects on microbes.
Figures

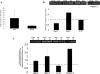

References
-
- Mun J. J., Tam C., Kowbel D., Hawgood S., Barnett M. J., Evans D. J. & Fleiszig S. M. Clearance of Pseudomonas aeruginosa from a healthy ocular surface involves surfactant protein D and is compromised by bacterial elastase in a murine null-infection model. Infect Immun. 77, 2392–8 (2009). - PMC - PubMed
-
- Fleiszig S. M., Lee E. J., Wu C., Andika R. C., Vallas V., Portoles M. & Frank D. W. Cytotoxic strains of Pseudomonas aeruginosa can damage the intact corneal surface in vitro. CLAO J. 24, 41–7 (1998). - PubMed

