Zic2 hypomorphic mutant mice as a schizophrenia model and ZIC2 mutations identified in schizophrenia patients
- PMID: 22355535
- PMCID: PMC3216504
- DOI: 10.1038/srep00016
Zic2 hypomorphic mutant mice as a schizophrenia model and ZIC2 mutations identified in schizophrenia patients
Erratum in
- Sci Rep.2011;1:49
Abstract
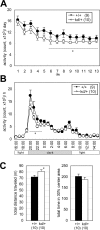
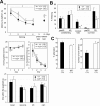
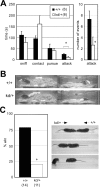
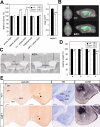
ZIC2 is a causal gene for holoprosencephaly and encodes a zinc-finger-type transcriptional regulator. We characterized Zic2(kd/+) mice with a moderate (40%) reduction in Zic2 expression. Zic2(kd/+) mice showed increased locomotor activity in novel environments, cognitive and sensorimotor gating dysfunctions, and social behavioral abnormalities. Zic2(kd/+) brain involved enlargement of the lateral ventricle, thinning of the cerebral cortex and corpus callosum, and decreased number of cholinergic neurons in the basal forebrain. Because these features are reminiscent of schizophrenia, we examined ZIC2 variant-carrying allele frequencies in schizophrenia patients and in controls in the Japanese population. Among three novel missense mutations in ZIC2, R409P was only found in schizophrenia patients, and was located in a strongly conserved position of the zinc finger domain. Mouse Zic2 with the corresponding mutation showed lowered transcription-activating capacity and had impaired target DNA-binding and co-factor-binding capacities. These results warrant further study of ZIC2 in the pathogenesis of schizophrenia.
Figures

References
-
- Aruga J. et al. The mouse zic gene family. Homologues of the Drosophila pair-rule gene odd-paired.J Biol Chem 271, 1043–1047 (1996). - PubMed
-
- Nagai T. et al. The expression of the mouse Zic1, Zic2, and Zic3 gene suggests an essential role for Zic genes in body pattern formation. Dev Biol 182, 299–313 (1997). - PubMed
-
- Aruga J. The role of Zic genes in neural development. Mol Cell Neurosci 26, 205–221 (2004). - PubMed
-
- Grinberg I. & Millen K. J. The ZIC gene family in development and disease. Clin Genet 67, 290–296, 10.1111/j.1399-0004.2005.00418.x (2005). - PubMed
-
- Merzdorf C. S. Emerging roles for zic genes in early development. Dev Dyn 236, 922–940 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

