Hyperthermic seizures and aberrant cellular homeostasis in Drosophila dystrophic muscles
- PMID: 22355566
- PMCID: PMC3216534
- DOI: 10.1038/srep00047
Hyperthermic seizures and aberrant cellular homeostasis in Drosophila dystrophic muscles
Abstract
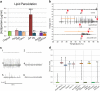
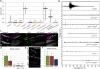
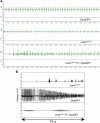
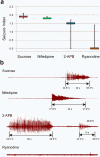
In humans, mutations in the Dystrophin Glycoprotein Complex (DGC) cause muscular dystrophies (MDs) that are associated with muscle loss, seizures and brain abnormalities leading to early death. Using Drosophila as a model to study MD we have found that loss of Dystrophin (Dys) during development leads to heat-sensitive abnormal muscle contractions that are repressed by mutations in Dys's binding partner, Dystroglycan (Dg). Hyperthermic seizures are independent from dystrophic muscle degeneration and rely on neurotransmission, which suggests involvement of the DGC in muscle-neuron communication. Additionally, reduction of the Ca(2+) regulator, Calmodulin or Ca(2+) channel blockage rescues the seizing phenotype, pointing to Ca(2+) mis-regulation in dystrophic muscles. Also, Dys and Dg mutants have antagonistically abnormal cellular levels of ROS, suggesting that the DGC has a function in regulation of muscle cell homeostasis. These data show that muscles deficient for Dys are predisposed to hypercontraction that may result from abnormal neuromuscular junction signaling.
Figures

Similar articles
-
Dg-Dys-Syn1 signaling in Drosophila regulates the microRNA profile.BMC Cell Biol. 2012 Oct 29;13:26. doi: 10.1186/1471-2121-13-26. BMC Cell Biol. 2012. PMID: 23107381 Free PMC article.
-
New dystrophin/dystroglycan interactors control neuron behavior in Drosophila eye.BMC Neurosci. 2011 Sep 26;12:93. doi: 10.1186/1471-2202-12-93. BMC Neurosci. 2011. PMID: 21943192 Free PMC article.
-
Muscle dystroglycan organizes the postsynapse and regulates presynaptic neurotransmitter release at the Drosophila neuromuscular junction.PLoS One. 2008 Apr 30;3(4):e2084. doi: 10.1371/journal.pone.0002084. PLoS One. 2008. PMID: 18446215 Free PMC article.
-
The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies.Muscle Nerve. 2001 Dec;24(12):1575-94. doi: 10.1002/mus.1192. Muscle Nerve. 2001. PMID: 11745966 Review.
-
The dystrophin-glycoprotein complex in the prevention of muscle damage.J Biomed Biotechnol. 2011;2011:210797. doi: 10.1155/2011/210797. Epub 2011 Oct 5. J Biomed Biotechnol. 2011. PMID: 22007139 Free PMC article. Review.
Cited by
-
Dg-Dys-Syn1 signaling in Drosophila regulates the microRNA profile.BMC Cell Biol. 2012 Oct 29;13:26. doi: 10.1186/1471-2121-13-26. BMC Cell Biol. 2012. PMID: 23107381 Free PMC article.
-
miRNA-based buffering of the cobblestone-lissencephaly-associated extracellular matrix receptor dystroglycan via its alternative 3'-UTR.Nat Commun. 2014 Sep 18;5:4906. doi: 10.1038/ncomms5906. Nat Commun. 2014. PMID: 25232965 Free PMC article.
-
Exocyst-mediated membrane trafficking of the lissencephaly-associated ECM receptor dystroglycan is required for proper brain compartmentalization.Elife. 2021 Feb 23;10:e63868. doi: 10.7554/eLife.63868. Elife. 2021. PMID: 33620318 Free PMC article.
-
Profiling of the muscle-specific dystroglycan interactome reveals the role of Hippo signaling in muscular dystrophy and age-dependent muscle atrophy.BMC Med. 2020 Jan 21;18(1):8. doi: 10.1186/s12916-019-1478-3. BMC Med. 2020. PMID: 31959160 Free PMC article.
-
New dystrophin/dystroglycan interactors control neuron behavior in Drosophila eye.BMC Neurosci. 2011 Sep 26;12:93. doi: 10.1186/1471-2202-12-93. BMC Neurosci. 2011. PMID: 21943192 Free PMC article.
References
-
- Barresi R. & Campbell K. P. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci 119, 199–207 (2006). - PubMed
-
- Grady R. M. et al.. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin–glycoprotein complex. Neuron 25, 279–293, S0896-6273(00)80894-6 [pii] (2000). - PubMed
-
- Kanagawa M. & Toda T. The genetic and molecular basis of muscular dystrophy: roles of cell-matrix linkage in the pathogenesis. J Hum Genet 51, 915–926, 10.1007/s10038-006-0056-7 (2006). - PubMed
-
- Sciandra F. et al.. Dystroglycan and muscular dystrophies related to the dystrophin-glycoprotein complex. Ann Ist Super Sanita 39, 173–181 (2003). - PubMed
-
- Lim L. E. & Campbell K. P. The sarcoglycan complex in limb-girdle muscular dystrophy. Curr.Opin.Neurol. 11, 443–452 (1998). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

