Population diversity and function of hyperpolarization-activated current in olfactory bulb mitral cells
- PMID: 22355569
- PMCID: PMC3216537
- DOI: 10.1038/srep00050
Population diversity and function of hyperpolarization-activated current in olfactory bulb mitral cells
Abstract
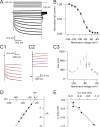
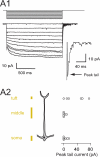
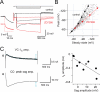
Although neurons are known to exhibit a broad array of intrinsic properties that impact critically on the computations they perform, very few studies have quantified such biophysical diversity and its functional consequences. Using in vivo and in vitro whole-cell recordings here we show that mitral cells are extremely heterogeneous in their expression of a rebound depolarization (sag) at hyperpolarized potentials that is mediated by a ZD7288-sensitive current with properties typical of hyperpolarization-activated cyclic nucleotide gated (HCN) channels. The variability in sag expression reflects a functionally diverse population of mitral cells. For example, those cells with large amplitude sag exhibit more membrane noise, a lower rheobase and fire action potentials more regularly than cells where sag is absent. Thus, cell-to-cell variability in sag potential amplitude reflects diversity in the integrative properties of mitral cells that ensures a broad dynamic range for odor representation across these principal neurons.
Figures
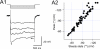



References
-
- Williams S. R. & Stuart G. J. Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J. Neurophysiol. 83, 3177–82 (2000). - PubMed
-
- Berger T., Larkum M. E. & Luscher H. R. High I(h) channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J. Neurophysiol. 85, 855–68 (2001). - PubMed
-
- Nolan M. F. et al.. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell 115, 551–64 (2003). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

