β-catenin is a molecular switch that regulates transition of cell-cell adhesion to fusion
- PMID: 22355587
- PMCID: PMC3216555
- DOI: 10.1038/srep00068
β-catenin is a molecular switch that regulates transition of cell-cell adhesion to fusion
Abstract
When a sperm and an oocyte unite upon fertilization, their cell membranes adhere and fuse, but little is known about the factors regulating sperm-oocyte adhesion. Here we explored the role of β-catenin in sperm-oocyte adhesion. Biochemical analysis revealed that E-cadherin and β-catenin formed a complex in oocytes and also in sperm. Sperm-oocyte adhesion was impaired when β-catenin-deficient oocytes were inseminated with sperm. Furthermore, expression of β-catenin decreased from the sperm head and the site of an oocyte to which a sperm adheres after completion of sperm-oocyte adhesion. UBE1-41, an inhibitor of ubiquitin-activating enzyme 1, inhibited the degradation of β-catenin, and reduced the fusing ability of wild-type (but not β-catenin-deficient) oocytes. These results indicate that β-catenin is not only involved in membrane adhesion, but also in the transition to membrane fusion upon fertilization.
Figures



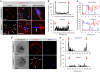
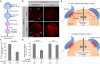
References
-
- Inoue N., Ikawa M., Isotani A., and Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434, 234–238 (2005). - PubMed
-
- Miyado K. et al.. Requirement of CD9 on the egg plasma membrane for fertilization. Science 287, 321–324 (2000). - PubMed
-
- Le Naour F. et al.., Severely reduced female fertility in CD9-deficient mice. Science 287, 319–321 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

