Mobile FT mRNA contributes to the systemic florigen signalling in floral induction
- PMID: 22355592
- PMCID: PMC3216560
- DOI: 10.1038/srep00073
Mobile FT mRNA contributes to the systemic florigen signalling in floral induction
Abstract
In inducing photoperiodic conditions, plants produce a signal dubbed "florigen" in leaves. Florigen moves through the phloem to the shoot apical meristem (SAM) where it induces flowering. In Arabidopsis, the FLOWERING LOCUS T (FT) protein acts as a component of this phloem-mobile signal. However whether the transportable FT mRNA also contributes to systemic florigen signalling remains to be elucidated. Using non-conventional approaches that exploit virus-induced RNA silencing and meristem exclusion of virus infection, we demonstrated that the ArabidopsisFT mRNA, independent of the FT protein, can move into the SAM. Viral ectopic expression of a non-translatable FT mRNA promoted earlier flowering in the short-day (SD) Nicotiana tabacum Maryland Mammoth tobacco in SD. These data suggest a possible role for FT mRNA in systemic floral signalling, and also demonstrate that cis-transportation of cellular mRNA into SAM and meristem exclusion of pathogenic RNAs are two mechanistically distinct processes.
Figures
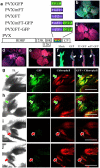

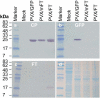
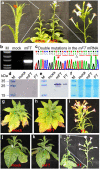

References
-
- Zeevaart J. A. D. Physiology of flower formation. Annu. Rev. Plant Physiol. 27, 321–348 (1976).
-
- Corbesier L. & Coupland G. The quest for florigen: a review of recent progress. J. Exp. Bot. 57, 3395–3403 (2006). - PubMed
-
- Abe M. et al.. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056 (2005). - PubMed
-
- An H. et al.. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131, 3615–3526 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

