Specific Y14 domains mediate its nucleo-cytoplasmic shuttling and association with spliced mRNA
- PMID: 22355610
- PMCID: PMC3216578
- DOI: 10.1038/srep00092
Specific Y14 domains mediate its nucleo-cytoplasmic shuttling and association with spliced mRNA
Abstract
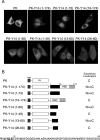
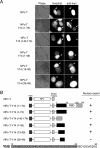
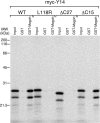
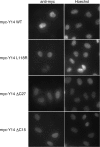
Pre-mRNA splicing deposits multi-protein complexes, termed exon junction complexes (EJCs), on mRNAs near exon-exon junctions. The core of EJC consists of four proteins, eIF4AIII, MLN51, Y14 and Magoh. Y14 is a nuclear protein that can shuttle between the nucleus and the cytoplasm, and binds specifically to Magoh. Here we delineate a Y14 nuclear localization signal that also confers its nuclear export, which we name YNS. We further identified a 12-amino-acid peptide near Y14's carboxyl terminus that is required for its association with spliced mRNAs, as well as for Magoh binding. Furthermore, the Y14 mutants, which are deficient in binding to Magoh, could still be localized to the nucleus, suggesting the existence of both the nuclear import pathway and function for Y14 unaccompanied by Magoh.
Figures

Similar articles
-
Association of the breast cancer protein MLN51 with the exon junction complex via its speckle localizer and RNA binding module.J Biol Chem. 2004 Aug 6;279(32):33702-15. doi: 10.1074/jbc.M402754200. Epub 2004 May 27. J Biol Chem. 2004. PMID: 15166247
-
Mutations equivalent to Drosophila mago nashi mutants imply reduction of Magoh protein incorporation into exon junction complex.Genes Cells. 2022 Jul;27(7):505-511. doi: 10.1111/gtc.12941. Epub 2022 Apr 25. Genes Cells. 2022. PMID: 35430764
-
Trypanosomes lack a canonical EJC but possess an UPF1 dependent NMD-like pathway.PLoS One. 2025 Mar 7;20(3):e0315659. doi: 10.1371/journal.pone.0315659. eCollection 2025. PLoS One. 2025. PMID: 40053537 Free PMC article.
-
Multifaceted roles of MAGOH Proteins.Mol Biol Rep. 2023 Feb;50(2):1931-1941. doi: 10.1007/s11033-022-07904-1. Epub 2022 Nov 17. Mol Biol Rep. 2023. PMID: 36396768 Review.
-
Nuclear mRNA binding proteins couple pre-mRNA splicing and post-splicing events.Mol Cells. 2001 Aug 31;12(1):1-10. Mol Cells. 2001. PMID: 11561715 Review.
Cited by
-
RNA-binding protein RBM8A (Y14) and MAGOH localize to centrosome in human A549 cells.Histochem Cell Biol. 2014 Jan;141(1):101-9. doi: 10.1007/s00418-013-1135-4. Epub 2013 Aug 15. Histochem Cell Biol. 2014. PMID: 23949737
-
A specific set of exon junction complex subunits is required for the nuclear retention of unspliced RNAs in Caenorhabditis elegans.Mol Cell Biol. 2013 Jan;33(2):444-56. doi: 10.1128/MCB.01298-12. Epub 2012 Nov 12. Mol Cell Biol. 2013. PMID: 23149939 Free PMC article.
-
Phosphorylation status of human RNA-binding protein 8A in cells and its inhibitory regulation by Magoh.Exp Biol Med (Maywood). 2015 Apr;240(4):438-45. doi: 10.1177/1535370214556945. Epub 2014 Oct 27. Exp Biol Med (Maywood). 2015. PMID: 25349214 Free PMC article.
-
Dual mechanisms regulate the nucleocytoplasmic localization of human DDX6.Sci Rep. 2017 Feb 20;7:42853. doi: 10.1038/srep42853. Sci Rep. 2017. PMID: 28216671 Free PMC article.
-
Function and pathological implications of exon junction complex factor Y14.Biomolecules. 2015 Apr 10;5(2):343-55. doi: 10.3390/biom5020343. Biomolecules. 2015. PMID: 25866920 Free PMC article. Review.
References
-
- Kataoka N., et al.. Pre-mRNA splicing imprints mRNA in the nucleus with a novel RNA-binding protein that persists in the cytoplasm. Mol. Cell 6, 673-682 (2000). - PubMed
-
- Shibuya T., Tange T. O., Sonenberg N. & Moore M. J. eIF4AIII binds spliced mRNA in the exon junction complex and is essential for nonsense-mediated decay. Nat. Struct. Mol. Biol. 11, 346-351 (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

