Synergistic interactions between kainate and mGlu receptors regulate bouton Ca signalling and mossy fibre LTP
- PMID: 22355621
- PMCID: PMC3216588
- DOI: 10.1038/srep00103
Synergistic interactions between kainate and mGlu receptors regulate bouton Ca signalling and mossy fibre LTP
Abstract
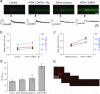
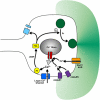
It is currently unknown why glutamatergic presynaptic terminals express multiple types of glutamate receptors. We have addressed this question by studying both acute and long-term regulation of mossy fibre function in the hippocampus. We find that inhibition of both mGlu₁ and mGlu₅ receptors together can block the induction of mossy fibre LTP. Furthermore, mossy fibre LTP can be induced by the pharmacological activation of either mGlu₁ or mGlu₅ receptors, provided that kainate receptors are also stimulated. Like conventional mossy fibre LTP, chemically-induced mossy fibre LTP (chem-LTPm) depends on Ca²⁺ release from intracellular stores and the activation of PKA. Similar synergistic interactions between mGlu receptors and kainate receptors were observed at the level of Ca²⁺ signalling in individual giant mossy fibre boutons. Thus three distinct glutamate receptors interact, in both an AND and OR gate fashion, to regulate both immediate and long-term presynaptic function in the brain.
Figures

Similar articles
-
Kainate receptors and the induction of mossy fibre long-term potentiation.Philos Trans R Soc Lond B Biol Sci. 2003 Apr 29;358(1432):657-66. doi: 10.1098/rstb.2002.1216. Philos Trans R Soc Lond B Biol Sci. 2003. PMID: 12740111 Free PMC article. Review.
-
An interchangeable role for kainate and metabotropic glutamate receptors in the induction of rat hippocampal mossy fiber long-term potentiation in vivo.Hippocampus. 2015 Nov;25(11):1407-17. doi: 10.1002/hipo.22460. Epub 2015 Apr 25. Hippocampus. 2015. PMID: 25821051 Free PMC article.
-
Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus.Neuron. 2001 Jan;29(1):209-16. doi: 10.1016/s0896-6273(01)00191-x. Neuron. 2001. PMID: 11182092
-
Differences in kainate receptor involvement in hippocampal mossy fibre long-term potentiation depending on slice orientation.Neurochem Int. 2012 Sep;61(4):482-9. doi: 10.1016/j.neuint.2012.04.021. Epub 2012 Apr 27. Neurochem Int. 2012. PMID: 22564530
-
GLUK1 receptor antagonists and hippocampal mossy fiber function.Int Rev Neurobiol. 2009;85:13-27. doi: 10.1016/S0074-7742(09)85002-2. Int Rev Neurobiol. 2009. PMID: 19607958 Review.
Cited by
-
Ionotropic receptors at hippocampal mossy fibers: roles in axonal excitability, synaptic transmission, and plasticity.Front Neural Circuits. 2013 Jan 9;6:112. doi: 10.3389/fncir.2012.00112. eCollection 2012. Front Neural Circuits. 2013. PMID: 23316138 Free PMC article.
-
Impaired presynaptic long-term potentiation in the anterior cingulate cortex of Fmr1 knock-out mice.J Neurosci. 2015 Feb 4;35(5):2033-43. doi: 10.1523/JNEUROSCI.2644-14.2015. J Neurosci. 2015. PMID: 25653361 Free PMC article.
-
Presynaptic localization of GluK5 in rod photoreceptors suggests a novel function of high affinity glutamate receptors in the mammalian retina.PLoS One. 2017 Feb 24;12(2):e0172967. doi: 10.1371/journal.pone.0172967. eCollection 2017. PLoS One. 2017. PMID: 28235022 Free PMC article.
-
Axonal Kainate Receptors Modulate the Strength of Efferent Connectivity by Regulating Presynaptic Differentiation.Front Cell Neurosci. 2016 Jan 20;10:3. doi: 10.3389/fncel.2016.00003. eCollection 2016. Front Cell Neurosci. 2016. PMID: 26834558 Free PMC article.
-
Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain.Neuron. 2015 Jan 21;85(2):377-89. doi: 10.1016/j.neuron.2014.12.021. Epub 2014 Dec 31. Neuron. 2015. PMID: 25556835 Free PMC article.
References
-
- Bliss T. V. & Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993). - PubMed
-
- Harris E. W. & Cotman C. W. Long-term potentiation of guinea pig mossy fibre responses is not blocked by N-methyl D-aspartate antagonists. Neurosci. Lett. 70, 132–137 (1986). - PubMed
-
- Nicoll R. A. & Malenka R. C. Contrasting properties of two forms of long term potentiation in the hippocampus. Nature 377, 115–118 (1995). - PubMed
-
- Bashir Z. I. et al.. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature 363, 347–350 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

