A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development
- PMID: 22355635
- PMCID: PMC3216599
- DOI: 10.1038/srep00118
A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development
Abstract
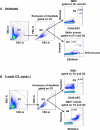
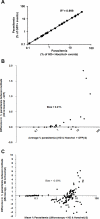
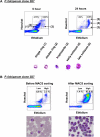
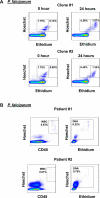
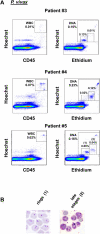
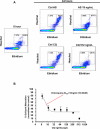
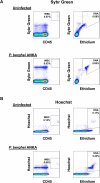
Microscopic examination of Giemsa-stained thin blood smears remains the gold standard method used to quantify and stage malaria parasites. However, this technique is tedious, and requires trained microscopists. We have developed a fast and simple flow cytometry method to quantify and stage, various malaria parasites in red blood cells in whole blood or in vitro cultured Plasmodium falciparum. The parasites were stained with dihydroethidium and Hoechst 33342 or SYBR Green I and leukocytes were identified with an antibody against CD45. Depending on the DNA stains used, samples were analyzed using different models of flow cytometers. This protocol, which does not require any washing steps, allows infected red blood cells to be distinguished from leukocytes, as well as allowing non-infected reticulocytes and normocytes to be identified. It also allows assessing the proportion of parasites at different developmental stages. Lastly, we demonstrate how this technique can be applied to antimalarial drug testing.
Figures

References
-
- Laveran A. Note sur un nouveau parasite trouve dans le sang de plusieurs malades atteints de fievre palustre. Bull Acad Med 9, 1235–1236 (1880).
-
- Giemsa G. Eine Vereinfachung und Vervollkommnung meiner Methylenazur-Methylenblau-Eosin-Färbemethode zur Erzielung der Romanowsky-Nochtschen Chromatinfärbung. Centralblatt für Bakteriologie 37, 308–311 (1904).
-
- Myler P., Saul A., Mangan T. & Kidson C. An automated assay of merozoite invasion of erythrocytes using highly synchronized Plasmodium falciparum cultures. Aust J Exp Biol Med Sci 60, 83–89 (1982). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

