An N-methyl-D-aspartate receptor agonist facilitates sleep-independent synaptic plasticity associated with working memory capacity enhancement
- PMID: 22355644
- PMCID: PMC3216608
- DOI: 10.1038/srep00127
An N-methyl-D-aspartate receptor agonist facilitates sleep-independent synaptic plasticity associated with working memory capacity enhancement
Abstract
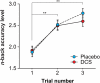
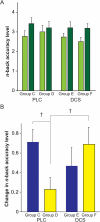
Working memory (WM) capacity improvement is impacted by sleep, and possibly by N-methyl-D-aspartate (NMDA) agonists such as D-cycloserine (DCS), which also affects procedural skill performance. However, the mechanisms behind these relationships are not well understood. In order to investigate the neural basis underlying relationships between WM skill learning and sleep, DCS, and both sleep and DCS together, we evaluated training-retest performances in the n-back task among healthy subjects who were given either a placebo or DCS before the task training, and then followed task training sessions either with wakefulness or sleep. DCS facilitated WM capacity enhancement only occurring after a period of wakefulness, rather than sleep, indicating that WM capacity enhancement is affected by a cellular heterogeneity in synaptic plasticity between time spent awake and time spent asleep. These findings may contribute to development, anti-aging processes, and rehabilitation of higher cognition.
Figures


Similar articles
-
Effects of Augmenting N-Methyl-D-Aspartate Receptor Signaling on Working Memory and Experience-Dependent Plasticity in Schizophrenia: An Exploratory Study Using Acute d-cycloserine.Schizophr Bull. 2017 Sep 1;43(5):1123-1133. doi: 10.1093/schbul/sbw193. Schizophr Bull. 2017. PMID: 28338977 Free PMC article. Clinical Trial.
-
D-cycloserine facilitates procedural learning but not declarative learning in healthy humans: a randomized controlled trial of the effect of D-cycloserine and valproic acid on overnight properties in the performance of non-emotional memory tasks.Neurobiol Learn Mem. 2011 May;95(4):505-9. doi: 10.1016/j.nlm.2011.02.017. Epub 2011 Mar 21. Neurobiol Learn Mem. 2011. PMID: 21402164 Clinical Trial.
-
Valproic acid but not D-cycloserine facilitates sleep-dependent offline learning of extinction and habituation of conditioned fear in humans.Neuropharmacology. 2013 Jan;64:424-31. doi: 10.1016/j.neuropharm.2012.07.045. Epub 2012 Aug 7. Neuropharmacology. 2013. PMID: 22992332 Clinical Trial.
-
D-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia.Schizophr Bull. 2012 Sep;38(5):936-41. doi: 10.1093/schbul/sbs012. Epub 2012 Feb 23. Schizophr Bull. 2012. PMID: 22368237 Free PMC article. Review.
-
D-cycloserine in Schizophrenia: New Strategies for Improving Clinical Outcomes by Enhancing Plasticity.Curr Neuropharmacol. 2017;15(1):21-34. doi: 10.2174/1570159x14666160225154812. Curr Neuropharmacol. 2017. PMID: 26915421 Free PMC article. Review.
Cited by
-
Nocturnal sleep enhances working memory training in Parkinson's disease but not Lewy body dementia.Brain. 2012 Sep;135(Pt 9):2789-97. doi: 10.1093/brain/aws192. Epub 2012 Aug 20. Brain. 2012. PMID: 22907117 Free PMC article.
-
The circadian regulation of sleep: impact of a functional ADA-polymorphism and its association to working memory improvements.PLoS One. 2014 Dec 1;9(12):e113734. doi: 10.1371/journal.pone.0113734. eCollection 2014. PLoS One. 2014. PMID: 25437848 Free PMC article.
-
Psychostimulants and atomoxetine alter the electrophysiological activity of prefrontal cortex neurons, interaction with catecholamine and glutamate NMDA receptors.Psychopharmacology (Berl). 2015 Jun;232(12):2191-205. doi: 10.1007/s00213-014-3849-y. Epub 2015 Jan 10. Psychopharmacology (Berl). 2015. PMID: 25572531
-
Endogenous IL-1 in cognitive function and anxiety: a study in IL-1RI-/- mice.PLoS One. 2013 Oct 30;8(10):e78385. doi: 10.1371/journal.pone.0078385. eCollection 2013. PLoS One. 2013. PMID: 24205219 Free PMC article.
-
The Role of N-Methyl-D-Aspartate Receptor Neurotransmission and Precision Medicine in Behavioral and Psychological Symptoms of Dementia.Front Pharmacol. 2019 May 22;10:540. doi: 10.3389/fphar.2019.00540. eCollection 2019. Front Pharmacol. 2019. PMID: 31191302 Free PMC article. Review.
References
-
- Baddeley A. D. & Hitch G. J. Working memory: The psychology of learning and motivation. (Bower G. A., ed) pp 47–89. New York: Academic Press (1974).
-
- Goldman-Rakic P. S. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos .Trans. R. Soc. Lond. B. Biol. Sci. 351, 1445–1453 (1996). - PubMed
-
- Owen A. M., Evans A. C. & Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb. Cortex 6, 31–38 (1996). - PubMed
-
- Fry A. F. & Hale S. Processing speed, working memory and fluid intelligence: evidence for a developmental cascade. Psychol. Sci. 7, 237–241 (1996).
-
- Hale S., Bronik M. D. & Fry A. F. Verbal and spatial working memory in school-age children: developmental differences in susceptibility to interference. Dev. Psychol. 33, 364–371 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

