Huntingtin mediates dendritic transport of β-actin mRNA in rat neurons
- PMID: 22355657
- PMCID: PMC3216621
- DOI: 10.1038/srep00140
Huntingtin mediates dendritic transport of β-actin mRNA in rat neurons
Abstract
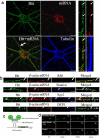
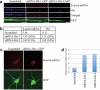
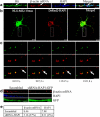
Transport of mRNAs to diverse neuronal locations via RNA granules serves an important function in regulating protein synthesis within restricted sub-cellular domains. We recently detected the Huntington's disease protein huntingtin (Htt) in dendritic RNA granules; however, the functional significance of this localization is not known. Here we report that Htt and the huntingtin-associated protein 1 (HAP1) are co-localized with the microtubule motor proteins, the KIF5A kinesin and dynein, during dendritic transport of β-actin mRNA. Live cell imaging demonstrated that β-actin mRNA is associated with Htt, HAP1, and dynein intermediate chain in cultured neurons. Reduction in the levels of Htt, HAP1, KIF5A, and dynein heavy chain by lentiviral-based shRNAs resulted in a reduction in the transport of β-actin mRNA. These findings support a role for Htt in participating in the mRNA transport machinery that also contains HAP1, KIF5A, and dynein.
Figures




References
-
- Gauthier L. R. et al.. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118, 127–138 (2004). - PubMed
-
- Gunawardena S. et al.. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron 40, 25–40 (2003). - PubMed
-
- Bramham C. R. & Wells D. G. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci 8, 776–789 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

