SIRT1 associates with eIF2-alpha and regulates the cellular stress response
- PMID: 22355666
- PMCID: PMC3252071
- DOI: 10.1038/srep00150
SIRT1 associates with eIF2-alpha and regulates the cellular stress response
Abstract
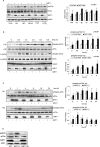
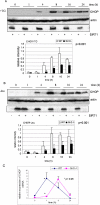
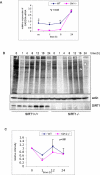
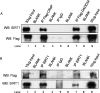
SIRT1 is a NAD+ dependent protein deacetylase known to increase longevity in model organisms. SIRT1 regulates cellular response to oxidative and/or genotoxic stress by regulating proteins such as p53 and FOXO. The eukaryotic initiation factor-2, eIF2, plays a critical role in the integrated stress response pathway. Under cellular stress, phosphorylation of the alpha subunit of eIF2 is essential for immediate shut-off of translation and activation of stress response genes. Here we demonstrate that SIRT1 interacts with eIF2α. Loss of SIRT1 results in increased phosphorylation of eIF2α. However, the downstream stress induced signaling pathway is compromised in SIRT1-deficient cells, indicated by delayed expression of the downstream target genes CHOP and GADD34 and a slower post-stress translation recovery. Finally, SIRT1 co-immunoprecipitates with mediators of eIF2α dephosphorylation, GADD34 and CreP, suggesting a role for SIRT1 in the negative feedback regulation of eIF2α phosphorylation.
Figures


Similar articles
-
Nck in a complex containing the catalytic subunit of protein phosphatase 1 regulates eukaryotic initiation factor 2alpha signaling and cell survival to endoplasmic reticulum stress.J Biol Chem. 2006 Sep 8;281(36):26633-44. doi: 10.1074/jbc.M513556200. Epub 2006 Jul 11. J Biol Chem. 2006. PMID: 16835242
-
Oxidative stress promotes SIRT1 recruitment to the GADD34/PP1α complex to activate its deacetylase function.Cell Death Differ. 2018 Feb;25(2):255-267. doi: 10.1038/cdd.2017.152. Epub 2017 Oct 6. Cell Death Differ. 2018. PMID: 28984870 Free PMC article.
-
SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation.Cell Death Differ. 2017 Feb;24(2):343-356. doi: 10.1038/cdd.2016.138. Epub 2016 Dec 2. Cell Death Differ. 2017. PMID: 27911441 Free PMC article.
-
The PPP1R15 Family of eIF2-alpha Phosphatase Targeting Subunits (GADD34 and CReP).Int J Mol Sci. 2023 Dec 10;24(24):17321. doi: 10.3390/ijms242417321. Int J Mol Sci. 2023. PMID: 38139150 Free PMC article. Review.
-
An Overview of Methods for Detecting eIF2α Phosphorylation and the Integrated Stress Response.Methods Mol Biol. 2022;2428:3-18. doi: 10.1007/978-1-0716-1975-9_1. Methods Mol Biol. 2022. PMID: 35171470 Review.
Cited by
-
Deficiency of CC chemokine ligand 2 and decay-accelerating factor causes retinal degeneration in mice.Exp Eye Res. 2015 Sep;138:126-33. doi: 10.1016/j.exer.2015.05.016. Epub 2015 Jul 3. Exp Eye Res. 2015. PMID: 26149093 Free PMC article.
-
Silencing of the ER and Integrative Stress Responses in the Liver of Mice with Error-Prone Translation.Cells. 2021 Oct 23;10(11):2856. doi: 10.3390/cells10112856. Cells. 2021. PMID: 34831079 Free PMC article.
-
Sirtuin1 and autophagy protect cells from fluoride-induced cell stress.Biochim Biophys Acta. 2014 Feb;1842(2):245-55. doi: 10.1016/j.bbadis.2013.11.023. Epub 2013 Dec 1. Biochim Biophys Acta. 2014. PMID: 24296261 Free PMC article.
-
Inactivation of Sirt1 in mouse livers protects against endotoxemic liver injury by acetylating and activating NF-κB.Cell Death Dis. 2016 Oct 6;7(10):e2403. doi: 10.1038/cddis.2016.270. Cell Death Dis. 2016. PMID: 27711079 Free PMC article.
-
SIRT1 activation promotes energy homeostasis and reprograms liver cancer metabolism.J Transl Med. 2023 Sep 15;21(1):627. doi: 10.1186/s12967-023-04440-9. J Transl Med. 2023. PMID: 37715252 Free PMC article.
References
-
- Alcendor R. R. et al.. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100, 1512–21 (2007). - PubMed
-
- Sinclair D. A. & Guarente L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell 91, 1033–42 (1997). - PubMed
-
- Tissenbaum H. A. & Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–30 (2001). - PubMed
-
- Qin W. et al.. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem 281, 21745–54 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

