The regulatory role of long-term depression in juvenile and adult mouse ocular dominance plasticity
- PMID: 22355718
- PMCID: PMC3243757
- DOI: 10.1038/srep00203
The regulatory role of long-term depression in juvenile and adult mouse ocular dominance plasticity
Erratum in
- Sci Rep. 2013;3:1442. Taghibiglou, Changiz [added]
Abstract
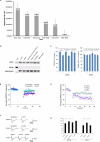
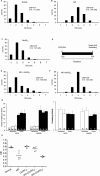
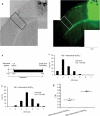
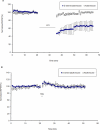
The study of experience-dependent ocular dominance (OD) plasticity has greatly contributed to the understanding of visual development. During the critical period, preventing input from one eye results in a significant impairment of vision, and loss of cortical responsivity via the deprived eye. Residual ocular dominance plasticity has recently been observed in adulthood. Accumulating evidence suggests that OD plasticity involves N-methyl-(D)-aspartate receptor (NMDAR)-dependent long-term depression (LTD). Here we report that the administration of a selective LTD antagonist prevented the ocular dominance shift during the critical period. The NMDAR co-agonist D-serine facilitated adult visual cortical LTD and the OD shift in short-term monocularly deprived (MD) adult mice. When combined with reverse suture, D-serine proved effective in restoring a contralaterally-dominated visual input pattern in long-term MD mice. This work suggests LTD as a key mechanism in both juvenile and adult ocular dominance plasticity, and D-serine as a potential therapeutic in human amblyopic subjects.
Figures

Similar articles
-
Essential role for a long-term depression mechanism in ocular dominance plasticity.Proc Natl Acad Sci U S A. 2009 Jun 16;106(24):9860-5. doi: 10.1073/pnas.0901305106. Epub 2009 May 22. Proc Natl Acad Sci U S A. 2009. PMID: 19470483 Free PMC article.
-
Arc restores juvenile plasticity in adult mouse visual cortex.Proc Natl Acad Sci U S A. 2017 Aug 22;114(34):9182-9187. doi: 10.1073/pnas.1700866114. Epub 2017 Aug 8. Proc Natl Acad Sci U S A. 2017. PMID: 28790183 Free PMC article.
-
Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex.J Neurosci. 2006 Mar 15;26(11):2951-5. doi: 10.1523/JNEUROSCI.5554-05.2006. J Neurosci. 2006. PMID: 16540572 Free PMC article.
-
Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex.Philos Trans R Soc Lond B Biol Sci. 2009 Feb 12;364(1515):357-67. doi: 10.1098/rstb.2008.0198. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 18977732 Free PMC article. Review.
-
Lifelong learning: ocular dominance plasticity in mouse visual cortex.Curr Opin Neurobiol. 2006 Aug;16(4):451-9. doi: 10.1016/j.conb.2006.06.007. Epub 2006 Jul 11. Curr Opin Neurobiol. 2006. PMID: 16837188 Review.
Cited by
-
Metabotropic glutamate receptor signaling is required for NMDA receptor-dependent ocular dominance plasticity and LTD in visual cortex.Proc Natl Acad Sci U S A. 2015 Oct 13;112(41):12852-7. doi: 10.1073/pnas.1512878112. Epub 2015 Sep 28. Proc Natl Acad Sci U S A. 2015. PMID: 26417096 Free PMC article.
-
The distinct role of NR2B subunit in the enhancement of visual plasticity in adulthood.Mol Brain. 2015 Aug 19;8:49. doi: 10.1186/s13041-015-0141-y. Mol Brain. 2015. PMID: 26282667 Free PMC article.
-
Recent Advances in the Molecular Mechanisms of Ocular Dominance Plasticity in the Visual Cortex.Neurosci Bull. 2025 Jun 24. doi: 10.1007/s12264-025-01448-7. Online ahead of print. Neurosci Bull. 2025. PMID: 40553377 Review.
-
The BCM theory of synapse modification at 30: interaction of theory with experiment.Nat Rev Neurosci. 2012 Nov;13(11):798-810. doi: 10.1038/nrn3353. Nat Rev Neurosci. 2012. PMID: 23080416 Review.
-
How the mechanisms of long-term synaptic potentiation and depression serve experience-dependent plasticity in primary visual cortex.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 2;369(1633):20130284. doi: 10.1098/rstb.2013.0284. Print 2014 Jan 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24298166 Free PMC article. Review.
References
-
- Hofer S. B., Mrsic-Flogel T. D., Bonhoeffer T. & Hubener M. Lifelong learning: ocular dominance plasticity in mouse visual cortex. Current opinion in neurobiology 16, 451–459 (2006). - PubMed
-
- Karmarkar U. R. & Dan Y. Experience-dependent plasticity in adult visual cortex. Neuron 52, 577–585 (2006). - PubMed
-
- Cynader M. & Mitchell D. E. Prolonged sensitivity to monocular deprivation in dark-reared cats. Journal of neurophysiology 43, 1026–1040 (1980). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

