Highly diastereoselective synthesis of tetrahydropyridines by a C-H activation-cyclization-reduction cascade
- PMID: 22356093
- PMCID: PMC3319108
- DOI: 10.1021/ja2119833
Highly diastereoselective synthesis of tetrahydropyridines by a C-H activation-cyclization-reduction cascade
Erratum in
- J Am Chem Soc. 2014 Aug 20;136(33):11849
Abstract
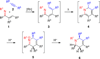
A versatile reaction cascade leading to highly substituted 1,2,3,6-tetrahydropyridines has been developed. It comprises rhodium(I)-catalyzed C-H activation-alkyne coupling followed by electrocyclization and subsequent acid/borohydride-promoted reduction. This one-pot procedure affords the target compounds in up to 95% yield with >95% diastereomeric purity.
© 2012 American Chemical Society
Figures
References
-
-
A large and increasing number of reports on the synthesis of heterocycles involving C–H activation has been published in the past few years. For recent examples, see the following publications and references therein: Jayakumar J, Parthasarathy K, Cheng C-H. Angew. Chem. Int. Ed. 2012;51:197–200. Yang G, Zhang W. Org. Lett. 2012;14:268–271. Mahoney SJ, Fillion E. Chem. Eur. J. 2012;18:68–71. Guimond N, Gorelsky SI, Fagnou K. J. Am. Chem. Soc. 2011;133:6449–6457. Huestis MP, Chan L, Stuart DR, Fagnou K. Angew. Chem. Int. Ed. 2011;50:1338–1341. Oberg KM, Rovis T. J. Am. Chem. Soc. 2011;133:4785–4787. Hyster TK, Rovis T. Chem. Sci. 2011;2:1606–1610. Du Y, Hyster TK, Rovis T. Chem. Commun. 2011;47:12074–12076. Morimoto K, Hirano K, Satoh T, Miura M. J. Org. Chem. 2011;76:9548–9551. Rakshit S, Grohmann C, Besset T, Glorius F. J. Am. Chem. Soc. 2011;133:2350–2353. Li X, Zhao M. J. Org. Chem. 2011;76:8530–8536. Shiota H, Ano Y, Aihara Y, Fukumoto Y, Chatani N. J. Am. Chem. Soc. 2011;133:14952–14955. Zhu C, Xie W, Falck JR. Chem. Eur. J. 2011;17:12591–12595.
-
-
-
For recent C–H activation reviews covering heterocycle syntheses, see: Engle KM, Mei T-S, Wasa M, Yu J-Q. Acc. Chem. Res. 2011 published online Dec. 14, 2011. Colby DA, Tsai AS, Bergman RG, Ellman JA. Acc. Chem. Res. 2011 published online Dec. 8, 2011. Yeung CS, Dong VM. Chem. Rev. 2011;111:1215–1292. Wencel-Delord J, Dröge T, Liu F, Glorius F. Chem. Soc. Rev. 2011;40:4740–4761. McMurray L, O’Hara F, Gaunt MJ. Chem. Soc. Rev. 2011;40:1885–1898. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147–1169. Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624–655. Xu L-M, Li B-J, Yang Z, Shi Z-J. Chem. Soc. Rev. 2010;39:712–733. Satoh T, Miura M. Chem. Eur. J. 2010;16:11212. Li C-J. Acc. Chem. Res. 2009;42:335–344. Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242–3272. Rubin M, Sromek AW, Gevorgyan V. Synlett. 2003;15:2265–2291.
-
-
-
For the synthesis of pyridines via C–H activation, see: Hyster TK, Rovis T. Chem. Commun. 2011;47:11846–11848. Colby DA, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2008;130:3645–3651. Parthasarathy K, Jeganmohan M, Cheng C-H. Org. Lett. 2008;10:325–328. For leading references on alternative multicomponent strategies for the synthesis of pyridines, see Chen Ming. Z, Micalizio GC. J. Am. Chem. Soc. 2012;134:1352–1356. Trost BM, Gutierrez AC. Org. Lett. 2007;9:1473–1476. Yamamoto Y, Kinpara K, Ogawa R, Nishiyama H, Itoh K. Chem. Eur. J. 2006;12:5618–5631. Movassaghi M, Hill MD. J. Am. Chem. Soc. 2006;128:4592–4593. McCormick MM, Duong HA, Zuo G, Louie J. J. Am. Chem. Soc. 2005;127:5030–5031. Tanaka R, Yuza A, Watai Y, Suzuki D, Takayama Y, Sato F, Urabe H. J. Am. Chem. Soc. 2005;127:7774–7780. Takahashi T, Tsai F-Y, Li Y, Wang H, Kondo Y, Yamanaka M, Nakajima K, Kotora M. J. Am. Chem. Soc. 2002;124:5059–5067.
-
-
-
For leading references on piperidines, see: Fattorusson E, Taglialatela-Scafati O, editors. Modern Alkaloids: Structure, Isolation, Synthesis and Biology. Wiley-CH: Weinheim; 2007. Michael JP. Nat. Prod. Rep. 2008;25:165. and earlier articles of this series. Mitchinson A, Nadin A. J. Chem. Soc. Perkin Trans. 2000;1:2862–2892. Laschat S, Dickner T. Synthesis. 2000;13:1781–1813.
-
-
-
For reviews on the chemistry and biological activity of tetrahydropyridines, see: Mateeva NN, Winfield LL, Redda KK. Curr. Med. Chem. 2005;12:551–571. Felpin F-X, Lebreton J. Curr. Org. Synth. 2004;1:83–109.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources