How do heme-protein sensors exclude oxygen? Lessons learned from cytochrome c', Nostoc puntiforme heme nitric oxide/oxygen-binding domain, and soluble guanylyl cyclase
- PMID: 22356101
- PMCID: PMC3430480
- DOI: 10.1089/ars.2012.4564
How do heme-protein sensors exclude oxygen? Lessons learned from cytochrome c', Nostoc puntiforme heme nitric oxide/oxygen-binding domain, and soluble guanylyl cyclase
Abstract
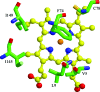
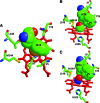
Significance: Ligand selectivity for dioxygen (O(2)), carbon monoxide (CO), and nitric oxide (NO) is critical for signal transduction and is tailored specifically for each heme-protein sensor. Key NO sensors, such as soluble guanylyl cyclase (sGC), specifically recognized low levels of NO and achieve a total O(2) exclusion. Several mechanisms have been proposed to explain the O(2) insensitivity, including lack of a hydrogen bond donor and negative electrostatic fields to selectively destabilize bound O(2), distal steric hindrance of all bound ligands to the heme iron, and restriction of in-plane movements of the iron atom.
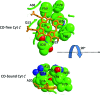
Recent advances: Crystallographic structures of the gas sensors, Thermoanaerobacter tengcongensis heme-nitric oxide/oxygen-binding domain (Tt H-NOX(1)) or Nostoc puntiforme (Ns) H-NOX, and measurements of O(2) binding to site-specific mutants of Tt H-NOX and the truncated β subunit of sGC suggest the need for a H-bonding donor to facilitate O(2) binding.
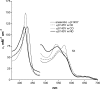
Critical issues: However, the O(2) insensitivity of full length sGC with a site-specific replacement of isoleucine by a tyrosine on residue 145 and the very slow autooxidation of Ns H-NOX and cytochrome c' suggest that more complex mechanisms have evolved to exclude O(2) but retain high affinity NO binding. A combined graphical analysis of ligand binding data for libraries of heme sensors, globins, and model heme shows that the NO sensors dramatically inhibit the formation of six-coordinated NO, CO, and O(2) complexes by direct distal steric hindrance (cyt c'), proximal constraints of in-plane iron movement (sGC), or combinations of both following a sliding scale rule. High affinity NO binding in H-NOX proteins is achieved by multiple NO binding steps that produce a high affinity five-coordinate NO complex, a mechanism that also prevents NO dioxygenation.
Future directions: Knowledge advanced by further extensive test of this "sliding scale rule" hypothesis should be valuable in guiding novel designs for heme based sensors.
Figures



Similar articles
-
Probing domain interactions in soluble guanylate cyclase.Biochemistry. 2011 May 24;50(20):4281-90. doi: 10.1021/bi200341b. Epub 2011 May 3. Biochemistry. 2011. PMID: 21491957 Free PMC article.
-
Insights into the distal heme pocket of H-NOX using fluoride as a probe for H-bonding interactions.J Inorg Biochem. 2013 Sep;126:91-5. doi: 10.1016/j.jinorgbio.2013.05.012. Epub 2013 Jun 3. J Inorg Biochem. 2013. PMID: 23792914
-
H-NOX domains display different tunnel systems for ligand migration.J Mol Graph Model. 2010 Jun;28(8):814-9. doi: 10.1016/j.jmgm.2010.02.007. Epub 2010 Mar 3. J Mol Graph Model. 2010. PMID: 20338794
-
Ligand discrimination in soluble guanylate cyclase and the H-NOX family of heme sensor proteins.Curr Opin Chem Biol. 2005 Oct;9(5):441-6. doi: 10.1016/j.cbpa.2005.08.015. Curr Opin Chem Biol. 2005. PMID: 16125437 Review.
-
A new paradigm for gaseous ligand selectivity of hemoproteins highlighted by soluble guanylate cyclase.J Inorg Biochem. 2021 Jan;214:111267. doi: 10.1016/j.jinorgbio.2020.111267. Epub 2020 Oct 16. J Inorg Biochem. 2021. PMID: 33099233 Free PMC article. Review.
Cited by
-
Spectral Characterization of a Novel NO Sensing Protein in Bacteria: NosP.Biochemistry. 2018 Oct 30;57(43):6187-6200. doi: 10.1021/acs.biochem.8b00451. Epub 2018 Oct 16. Biochemistry. 2018. PMID: 30272959 Free PMC article.
-
YC-1 binding to the β subunit of soluble guanylyl cyclase overcomes allosteric inhibition by the α subunit.Biochemistry. 2014 Jan 14;53(1):101-14. doi: 10.1021/bi4015133. Epub 2013 Dec 30. Biochemistry. 2014. PMID: 24328155 Free PMC article.
-
Chemical foundations of hydrogen sulfide biology.Nitric Oxide. 2013 Nov 30;35:21-34. doi: 10.1016/j.niox.2013.07.001. Epub 2013 Jul 9. Nitric Oxide. 2013. PMID: 23850631 Free PMC article. Review.
-
The Role of Reactive Oxygen and Nitrogen Species in the Expression and Splicing of Nitric Oxide Receptor.Antioxid Redox Signal. 2017 Jan 20;26(3):122-136. doi: 10.1089/ars.2016.6687. Epub 2016 Apr 19. Antioxid Redox Signal. 2017. PMID: 26972233 Free PMC article. Review.
-
Modulation of ligand-heme reactivity by binding pocket residues demonstrated in cytochrome c' over the femtosecond-second temporal range.FEBS J. 2013 Dec;280(23):6070-82. doi: 10.1111/febs.12526. Epub 2013 Oct 11. FEBS J. 2013. PMID: 24034856 Free PMC article.
References
-
- Akimoto S. Tanaka A. Nakamura K. Shiro Y. Nakamura H. O2-specific regulation of the ferrous heme-based sensor kinase FixL from Sinorhizobium meliloti and its aberrant inactivation in the ferric form. Biochem Biophys Res Commun. 2003;304:136–142. - PubMed
-
- Andrew CR. George SJ. Lawson DM. Eady RR. Six- to five-coordinate heme-nitrosyl conversion in cytochrome c′ and its relevance to guanylate cyclase. Biochemistry. 2002;41:2353–2360. - PubMed
-
- Andrew CR. Green EL. Lawson DM. Eady RR. Resonance Raman studies of cytochrome c′ support the binding of NO and CO to opposite sides of the heme: implications for ligand discrimination in heme-based sensors. Biochemistry. 2001;40:4115–4122. - PubMed
-
- Andrew CR. Kemper LJ. Busche TL. Tiwari AM. Kecskes MC. Stafford JM. Croft LC. Lu S. Moenne-Loccoz P. Huston W. Moir JW. Eady RR. Accessibility of the distal heme face, rather than Fe-His bond strength, determines the heme-nitrosyl coordination number of cytochromes c′: evidence from spectroscopic studies. Biochemistry. 2005;44:8664–8672. - PubMed
-
- Andrew CR. Rodgers KR. Eady RR. A novel kinetic trap for NO release from cytochrome c′: a possible mechanism for NO release from activated soluble guanylate cyclase. J Am Chem Soc. 2003;125:9548–9549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

