A single-site mutation (F429H) converts the enzyme CYP 2B4 into a heme oxygenase: a QM/MM study
- PMID: 22356576
- PMCID: PMC3326604
- DOI: 10.1021/ja211905e
A single-site mutation (F429H) converts the enzyme CYP 2B4 into a heme oxygenase: a QM/MM study
Abstract
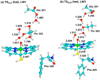
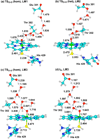
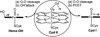
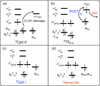
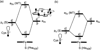
The intriguing deactivation of the cytochrome P450 (CYP) 2B4 enzyme induced by mutation of a single residue, Phe429 to His, is explored by quantum mechanical/molecular mechanical calculations of the O-OH bond activation of the (Fe(3+)OOH)(-) intermediate. It is found that the F429H mutant of CYP 2B4 undergoes homolytic instead of heterolytic O-OH bond cleavage. Thus, the mutant acquires the following characteristics of a heme oxygenase enzyme: (a) donation by His429 of an additional NH---S H-bond to the cysteine ligand combined with the presence of the substrate retards the heterolytic cleavage and gives rise to homolytic O-OH cleavage, and (b) the Thr302/water cluster orients nascent OH(•) and ensures efficient meso hydroxylation.
© 2012 American Chemical Society
Figures


References
-
- Ortiz de Montellano PR, editor. Cytochrome P450: structure, mechanism and biochemistry. 3rd ed. New York: Kluwer Academic/Plenum Publishers; 2004.
- Shaik S, Cohen S, Wang Y, Chen H, Kumar D, Thiel W. Chem. Rev. 2010;110:949–1017. - PubMed
-
- Sherwood P, et al. J. Mol. Struct. THEOCHEM. 2003;632:1–28.
- Brooks BR, Burccoleri RE, Olafson BD, States DJ, Karplus M. J. Comput. Chem. 1983;4:187–217.
- Ahlrichs R, Bär M, Häser M, Horn H, Kölmel C. Chem. Phys. Lett. 1989;162:165–169.
- Senn HM, Thiel W. Angew. Chem. Int. Ed. 2009;48:1198–1229. - PubMed
- Zheng J, Wang D, Thiel W, Shaik S. J. Am. Chem. Soc. 2006;128:13204–13214. - PubMed
- Chen H, Hirao H, Derat E, Schlichting I, Shaik S. J. Phys. Chem. B. 2008;112:9490–9500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

