Direct evidence of generation and accumulation of β-sheet-rich prion protein in scrapie-infected neuroblastoma cells with human IgG1 antibody specific for β-form prion protein
- PMID: 22356913
- PMCID: PMC3340157
- DOI: 10.1074/jbc.M111.318352
Direct evidence of generation and accumulation of β-sheet-rich prion protein in scrapie-infected neuroblastoma cells with human IgG1 antibody specific for β-form prion protein
Abstract
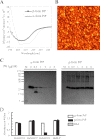
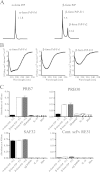
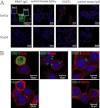
We prepared β-sheet-rich recombinant full-length prion protein (β-form PrP) (Jackson, G. S., Hosszu, L. L., Power, A., Hill, A. F., Kenney, J., Saibil, H., Craven, C. J., Waltho, J. P., Clarke, A. R., and Collinge, J. (1999) Science 283, 1935-1937). Using this β-form PrP and a human single chain Fv-displaying phage library, we have established a human IgG1 antibody specific to β-form but not α-form PrP, PRB7 IgG. When prion-infected ScN2a cells were cultured with PRB7 IgG, they generated and accumulated PRB7-binding granules in the cytoplasm with time, consequently becoming apoptotic cells bearing very large PRB7-bound aggregates. The SAF32 antibody recognizing the N-terminal octarepeat region of full-length PrP stained distinct granules in these cells as determined by confocal laser microscopy observation. When the accumulation of proteinase K-resistant PrP was examined in prion-infected ScN2a cells cultured in the presence of PRB7 IgG or SAF32, it was strongly inhibited by SAF32 but not at all by PRB7 IgG. Thus, we demonstrated direct evidence of the generation and accumulation of β-sheet-rich PrP in ScN2a cells de novo. These results suggest first that PRB7-bound PrP is not responsible for the accumulation of β-form PrP aggregates, which are rather an end product resulting in the triggering of apoptotic cell death, and second that SAF32-bound PrP lacking the PRB7-recognizing β-form may represent so-called PrP(Sc) with prion propagation activity. PRB7 is the first human antibody specific to β-form PrP and has become a powerful tool for the characterization of the biochemical nature of prion and its pathology.
Figures






Similar articles
-
Inhibition of prion propagation in scrapie-infected mouse neuroblastoma cell lines using mouse monoclonal antibodies against prion protein.Biochem Biophys Res Commun. 2005 Sep 16;335(1):197-204. doi: 10.1016/j.bbrc.2005.07.063. Biochem Biophys Res Commun. 2005. PMID: 16061207
-
Cooperative binding of dominant-negative prion protein to kringle domains.J Mol Biol. 2003 May 30;329(2):323-33. doi: 10.1016/s0022-2836(03)00342-5. J Mol Biol. 2003. PMID: 12758079
-
Disulfide-crosslink scanning reveals prion-induced conformational changes and prion strain-specific structures of the pathological prion protein PrPSc.J Biol Chem. 2018 Aug 17;293(33):12730-12740. doi: 10.1074/jbc.RA117.001633. Epub 2018 Jun 22. J Biol Chem. 2018. PMID: 29934306 Free PMC article.
-
Prion protein oligomer and its neurotoxicity.Acta Biochim Biophys Sin (Shanghai). 2013 Jun;45(6):442-51. doi: 10.1093/abbs/gmt037. Epub 2013 Apr 4. Acta Biochim Biophys Sin (Shanghai). 2013. PMID: 23557632 Review.
-
Prion protein and its conformational conversion: a structural perspective.Top Curr Chem. 2011;305:135-67. doi: 10.1007/128_2011_165. Top Curr Chem. 2011. PMID: 21630136 Review.
Cited by
-
Exposure to bacterial endotoxin generates a distinct strain of α-synuclein fibril.Sci Rep. 2016 Aug 4;6:30891. doi: 10.1038/srep30891. Sci Rep. 2016. PMID: 27488222 Free PMC article.
-
Role of lipid rafts and GM1 in the segregation and processing of prion protein.PLoS One. 2014 May 23;9(5):e98344. doi: 10.1371/journal.pone.0098344. eCollection 2014. PLoS One. 2014. PMID: 24859148 Free PMC article.
-
Prion protein-specific antibodies-development, modes of action and therapeutics application.Viruses. 2014 Oct 1;6(10):3719-37. doi: 10.3390/v6103719. Viruses. 2014. PMID: 25275428 Free PMC article. Review.
-
Single-chain fragment variable passive immunotherapies for neurodegenerative diseases.Int J Mol Sci. 2013 Sep 17;14(9):19109-27. doi: 10.3390/ijms140919109. Int J Mol Sci. 2013. PMID: 24048248 Free PMC article. Review.
References
-
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. (1983) Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35, 349–358 - PubMed
-
- Turk E., Teplow D. B., Hood L. E., Prusiner S. B. (1988) Purification and properties of the cellular and scrapie hamster prion proteins. Eur. J. Biochem. 176, 21–30 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

