Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems
- PMID: 22357101
- PMCID: PMC3341493
- DOI: 10.1016/j.freeradbiomed.2012.01.025
Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems
Abstract
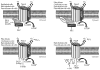
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are ubiquitously produced in cardiovascular systems. Under physiological conditions, ROS/RNS function as signaling molecules that are essential in maintaining cardiovascular function. Aberrant concentrations of ROS/RNS have been demonstrated in cardiovascular diseases owing to increased production or decreased scavenging, which have been considered common pathways for the initiation and progression of cardiovascular diseases such as atherosclerosis, hypertension, (re)stenosis, and congestive heart failure. NAD(P)H oxidases are primary sources of ROS and can be induced or activated by all known cardiovascular risk factors. Stresses, hormones, vasoactive agents, and cytokines via different signaling cascades control the expression and activity of these enzymes and of their regulatory subunits. But the molecular mechanisms by which NAD(P)H oxidase is regulated in cardiovascular systems remain poorly characterized. Investigations by us and others suggest that adenosine monophosphate-activated protein kinase (AMPK), as an energy sensor and modulator, is highly sensitive to ROS/RNS. We have also obtained convincing evidence that AMPK is a physiological suppressor of NAD(P)H oxidase in multiple cardiovascular cell systems. In this review, we summarize our current understanding of how AMPK functions as a physiological repressor of NAD(P)H oxidase.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. - PubMed
-
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. - PubMed
-
- Wosniak J, Jr, Santos CX, Kowaltowski AJ, Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal. 2009;11:1265–1278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

