Molecular evidence for natural killer-like cells in equine endometrial cups
- PMID: 22357194
- PMCID: PMC3319276
- DOI: 10.1016/j.placenta.2012.01.018
Molecular evidence for natural killer-like cells in equine endometrial cups
Abstract
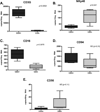
Objectives: To identify equine orthologs of major NK cell marker genes and utilize them to determine whether NK cells are present among the dense infiltration of lymphocytes that surround the endometrial cup structures of the horse placenta during early pregnancy.
Study design: PCR primers were developed to detect the equine orthologs of NKP46, CD16, CD56, and CD94; gene expression was detected in RNA isolated from lymphocytes using standard 2-step reverse transcriptase (RT) PCR and products were cloned and sequenced. Absolute real-time RT-PCR was used to quantitate gene expression in total, CD3+, and CD3- peripheral lymphocytes, and invasive trophoblast. Lymphocytes surrounding the endometrial cups (ECL) of five mares in early pregnancy were isolated and NK marker gene expression levels were assayed by quantitative RT-PCR.
Main outcome measures: Absolute mRNA transcript numbers were determined by performing quantitative RT-PCR and comparing values to plasmid standards of known quantities.
Results: NKP46 gene expression in peripheral CD3- lymphocytes was higher than in CD3+ lymphocytes, CD16 levels were higher in the CD3+ population, and no significant differences were detected for CD56 and CD94 between the two groups. Expression of all four NK cell markers was significantly higher in lymphocytes isolated from the endometrial cups of pregnant mares compared to PBMC isolated from the same animal on the same day (NKP46, 14-fold higher; CD94, 8-fold higher; CD16, 20-fold higher; CD56, 44-fold higher).
Conclusions: These data provide the first evidence for the expression of major NK cell markers by horse cells and an enrichment of NK-like cells in the equine endometrium during pregnancy.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Endometrial natural killer (NK) cells reveal a tissue-specific receptor repertoire.Hum Reprod. 2018 Mar 1;33(3):441-451. doi: 10.1093/humrep/dey001. Hum Reprod. 2018. PMID: 29447367
-
IL-22 is expressed by the invasive trophoblast of the equine (Equus caballus) chorionic girdle.J Immunol. 2012 May 1;188(9):4181-7. doi: 10.4049/jimmunol.1103509. Epub 2012 Apr 4. J Immunol. 2012. PMID: 22490443 Free PMC article.
-
Expression of natural cytotoxicity receptors and cytokine production on endometrial natural killer cells in women with recurrent pregnancy loss or implantation failure, and the expression of natural cytotoxicity receptors on peripheral blood natural killer cells in pregnant women with a history of recurrent pregnancy loss.J Obstet Gynaecol Res. 2017 Nov;43(11):1678-1686. doi: 10.1111/jog.13448. Epub 2017 Aug 17. J Obstet Gynaecol Res. 2017. PMID: 28815854
-
Maternal immunological recognition of pregnancy in equids.J Reprod Fertil Suppl. 1989;37:69-78. J Reprod Fertil Suppl. 1989. PMID: 2681748 Review.
-
Uterine natural killer cells in species with epitheliochorial placentation.Nat Immun. 1996-1997;15(1):53-69. Nat Immun. 1996. PMID: 9032768 Review.
Cited by
-
Generation and characterization of monoclonal antibodies to equine NKp46.Vet Immunol Immunopathol. 2012 Jun 15;147(1-2):60-8. doi: 10.1016/j.vetimm.2012.04.003. Epub 2012 Apr 7. Vet Immunol Immunopathol. 2012. PMID: 22551980 Free PMC article.
-
Evolution of Placental Hormones: Implications for Animal Models.Front Endocrinol (Lausanne). 2022 May 25;13:891927. doi: 10.3389/fendo.2022.891927. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35692413 Free PMC article. Review.
-
High Expression of Endogenous Retroviral Envelope Gene in the Equine Fetal Part of the Placenta.PLoS One. 2016 May 13;11(5):e0155603. doi: 10.1371/journal.pone.0155603. eCollection 2016. PLoS One. 2016. PMID: 27176223 Free PMC article.
-
Generation and characterization of monoclonal antibodies to equine CD16.Vet Immunol Immunopathol. 2012 Apr 15;146(2):135-42. doi: 10.1016/j.vetimm.2012.02.006. Epub 2012 Feb 23. Vet Immunol Immunopathol. 2012. PMID: 22424928 Free PMC article.
-
Major histocompatibility complex I mediates immunological tolerance of the trophoblast during pregnancy and may mediate rejection during parturition.Mediators Inflamm. 2014;2014:579279. doi: 10.1155/2014/579279. Epub 2014 Apr 9. Mediators Inflamm. 2014. PMID: 24812442 Free PMC article. Review.
References
-
- Bacon SJ, Ellis SA, Antczak DF. Control of expression of major histocompatibility complex genes in horse trophoblast. Biol Reprod. 2002;66(6):1612–1620. - PubMed
-
- Donaldson WL, Oriol JG, Pelkaus CL, Antczak DF. Paternal and maternal major histocompatibility complex class I antigens are expressed co-dominantly by equine trophoblast. Placenta. 1994;15(2):123–135. - PubMed
-
- Allen WR, Hamilton DW, Moor RM. The origin of equine endometrial cups. II. Invasion of the endometrium by trophoblast. Anat Rec. 1973;177(4):485–501. - PubMed
-
- Grunig G, Triplett L, Canady LK, Allen WR, Antczak DF. The maternal leucocyte response to the endometrial cups in horses is correlated with the developmental stages of the invasive trophoblast cells. Placenta. 1995;16(6):539–559. - PubMed
-
- Antczak DF, Miller JM, Remick LH. Lymphocyte alloantigens of the horse. II. Antibodies to ELA antigens produced during equine pregnancy. J Reprod Immunol. 1984;6(5):283–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

