Vpu augments the initial burst phase of HIV-1 propagation and downregulates BST2 and CD4 in humanized mice
- PMID: 22357275
- PMCID: PMC3347374
- DOI: 10.1128/JVI.07062-11
Vpu augments the initial burst phase of HIV-1 propagation and downregulates BST2 and CD4 in humanized mice
Abstract
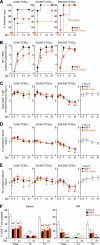
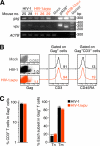
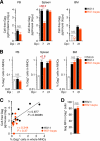
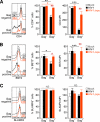
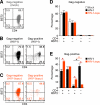
While human cells express potent antiviral proteins as part of the host defense repertoire, viruses have evolved their own arsenal of proteins to antagonize them. BST2 was identified as an inhibitory cellular protein of HIV-1 replication, which tethers virions to the cell surface to prevent their release. On the other hand, the HIV-1 accessory protein, Vpu, has the ability to downregulate and counteract BST2. Vpu also possesses the ability to downmodulate cellular CD4 and SLAMF6 molecules expressed on infected cells. However, the role of Vpu in HIV-1 infection in vivo remains unclear. Here, using a human hematopoietic stem cell-transplanted humanized mouse model, we demonstrate that Vpu contributes to the efficient spread of HIV-1 in vivo during the acute phase of infection. Although Vpu did not affect viral cytopathicity, target cell preference, and the level of viral protein expression, the amount of cell-free virions in vpu-deficient HIV-1-infected mice was profoundly lower than that in wild-type HIV-1-infected mice. We provide a novel insight suggesting that Vpu concomitantly downregulates BST2 and CD4, but not SLAMF6, from the surface of infected cells. Furthermore, we show evidence suggesting that BST2 and CD4 impair the production of cell-free infectious virions but do not associate with the efficiency of cell-to-cell HIV-1 transmission. Taken together, our findings suggest that Vpu downmodulates BST2 and CD4 in infected cells and augments the initial burst of HIV-1 replication in vivo. This is the first report demonstrating the role of Vpu in HIV-1 infection in an in vivo model.
Figures



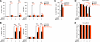
Similar articles
-
Efficient BST2 antagonism by Vpu is critical for early HIV-1 dissemination in humanized mice.Retrovirology. 2013 Nov 6;10:128. doi: 10.1186/1742-4690-10-128. Retrovirology. 2013. PMID: 24195843 Free PMC article.
-
Contribution of the Cytoplasmic Determinants of Vpu to the Expansion of Virus-Containing Compartments in HIV-1-Infected Macrophages.J Virol. 2019 May 15;93(11):e00020-19. doi: 10.1128/JVI.00020-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30867316 Free PMC article.
-
Activities of transmitted/founder and chronic clade B HIV-1 Vpu and a C-terminal polymorphism specifically affecting virion release.J Virol. 2014 May;88(9):5062-78. doi: 10.1128/JVI.03472-13. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574397 Free PMC article.
-
Modulation of HIV-1-host interaction: role of the Vpu accessory protein.Retrovirology. 2010 Dec 22;7:114. doi: 10.1186/1742-4690-7-114. Retrovirology. 2010. PMID: 21176220 Free PMC article. Review.
-
β-TrCP dependency of HIV-1 Vpu-induced downregulation of CD4 and BST-2/tetherin.Curr HIV Res. 2012 Jun;10(4):307-14. doi: 10.2174/157016212800792441. Curr HIV Res. 2012. PMID: 22524179 Review.
Cited by
-
Upregulation of BST-2 by Type I Interferons Reduces the Capacity of Vpu To Protect HIV-1-Infected Cells from NK Cell Responses.mBio. 2019 Jun 18;10(3):e01113-19. doi: 10.1128/mBio.01113-19. mBio. 2019. PMID: 31213558 Free PMC article.
-
HIV restriction by APOBEC3 in humanized mice.PLoS Pathog. 2013 Mar;9(3):e1003242. doi: 10.1371/journal.ppat.1003242. Epub 2013 Mar 28. PLoS Pathog. 2013. PMID: 23555255 Free PMC article.
-
Vpu and BST2: Still Not There Yet?Front Microbiol. 2012 Apr 9;3:131. doi: 10.3389/fmicb.2012.00131. eCollection 2012. Front Microbiol. 2012. PMID: 22509177 Free PMC article.
-
Quantification of deaminase activity-dependent and -independent restriction of HIV-1 replication mediated by APOBEC3F and APOBEC3G through experimental-mathematical investigation.J Virol. 2014 May;88(10):5881-7. doi: 10.1128/JVI.00062-14. Epub 2014 Mar 12. J Virol. 2014. PMID: 24623435 Free PMC article.
-
Cell Surface Proteomic Map of HIV Infection Reveals Antagonism of Amino Acid Metabolism by Vpu and Nef.Cell Host Microbe. 2015 Oct 14;18(4):409-23. doi: 10.1016/j.chom.2015.09.003. Epub 2015 Oct 1. Cell Host Microbe. 2015. PMID: 26439863 Free PMC article.
References
-
- Arganaraz ER, Schindler M, Kirchhoff F, Cortes MJ, Lama J. 2003. Enhanced CD4 down-modulation by late stage HIV-1 nef alleles is associated with increased Env incorporation and viral replication. J. Biol. Chem. 278:33912–33919 - PubMed
-
- Carr JM, Hocking H, Li P, Burrell CJ. 1999. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology 265:319–329 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

