Clinical validation of a point-of-care multiplexed in vitro immunoassay using monoclonal antibodies (the MSD influenza test) in four hospitals in Vietnam
- PMID: 22357497
- PMCID: PMC3347120
- DOI: 10.1128/JCM.00085-12
Clinical validation of a point-of-care multiplexed in vitro immunoassay using monoclonal antibodies (the MSD influenza test) in four hospitals in Vietnam
Abstract
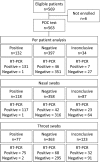
Point-of-care (POC) diagnostic tests for influenza can considerably shorten the time to clinical decision making. An investigational POC test based on a multiplexed immunoassay was developed by Meso Scale Diagnostics, LLC (MSD), with the objective to make a more sensitive rapid test that can also subtype influenza A viruses (1977 H1, H3, and H5). Between February and November 2010, we conducted a prospective multicenter study at four hospitals in Vietnam and compared the performance of this test to that of the WHO/CDC real-time reverse transcriptase PCR (RT-PCR) on nasal and throat swab specimens from patients presenting with influenza-like illness. Five hundred sixty-three adults and children with a median age of 25 months were enrolled. Sensitivity and specificity of the test with combined results from nasal and throat swab samples were 74.0% (131/177) and 99.7% (351/352), respectively, compared to RT-PCR. The POC test was as sensitive for influenza virus B as for influenza virus A (74.4% [64/86] versus 73.6% [67/91]). The positivity rate was associated with lower cycle threshold values (a marker for higher viral loads), sample type (73.6% for nasal swab versus 52.4% for throat swab), and younger age. A total of 210 (18.7%) out of 1,126 MSD tests failed, and for 34 (6%) of patients, both test samples failed (these were excluded from the performance analysis). Subtyping could be assessed only for influenza virus A/H3N2, as 1977 H1N1 was not circulating at the time and no H5N1-infected patients were enrolled, and was successful only in 9/54 patients infected with H3 influenza virus who had a positive POC test result for influenza virus A. This novel POC test provided highly sensitive detection of influenza viruses A and B compared to the reported sensitivities of other rapid tests. However, 18.7% of tests failed for technical reasons and subtyping for H3 was poor. Drawbacks to the technology include the requirement for a dedicated reader instrument and the need for continual updating of subtyping antibodies within the test array.
Figures
Similar articles
-
Evaluating the performance of a rapid antigen test for the detection of influenza virus in clinical specimens from children in Cameroon.Influenza Other Respir Viruses. 2014 Mar;8(2):131-4. doi: 10.1111/irv.12210. Epub 2013 Nov 24. Influenza Other Respir Viruses. 2014. PMID: 24266902 Free PMC article.
-
Evaluation of rapid influenza virus tests in patients with influenza-like illness in Thailand.Clin Lab. 2012;58(9-10):905-10. Clin Lab. 2012. PMID: 23163105 Clinical Trial.
-
Comparison of the performance of direct fluorescent antibody staining, a point-of-care rapid antigen test and virus isolation with that of RT-PCR for the detection of novel 2009 influenza A (H1N1) virus in respiratory specimens.J Med Microbiol. 2010 Jun;59(Pt 6):713-717. doi: 10.1099/jmm.0.017244-0. Epub 2010 Mar 4. J Med Microbiol. 2010. PMID: 20203216
-
Identification of respiratory viruses with a novel point-of-care multianalyte antigen detection test in children with acute respiratory tract infection.J Clin Virol. 2013 Jun;57(2):136-40. doi: 10.1016/j.jcv.2013.02.011. Epub 2013 Mar 11. J Clin Virol. 2013. PMID: 23490399 Free PMC article.
-
Comparison of the Roche RealTime ready Influenza A/H1N1 Detection Set with CDC A/H1N1pdm09 RT-PCR on samples from three hospitals in Ho Chi Minh City, Vietnam.Diagn Microbiol Infect Dis. 2012 Oct;74(2):131-6. doi: 10.1016/j.diagmicrobio.2012.06.003. Epub 2012 Jul 9. Diagn Microbiol Infect Dis. 2012. PMID: 22785431 Free PMC article.
Cited by
-
An integrated device for the rapid and sensitive detection of the influenza hemagglutinin.Lab Chip. 2019 Feb 26;19(5):885-896. doi: 10.1039/c8lc00691a. Lab Chip. 2019. PMID: 30724293 Free PMC article.
-
Evaluation of Alere i Influenza A&B for rapid detection of influenza viruses A and B.J Clin Microbiol. 2014 Sep;52(9):3339-44. doi: 10.1128/JCM.01132-14. Epub 2014 Jul 2. J Clin Microbiol. 2014. PMID: 24989611 Free PMC article.
-
Rapid Molecular Detection and Differentiation of Influenza Viruses A and B.J Vis Exp. 2017 Jan 30;(119):54312. doi: 10.3791/54312. J Vis Exp. 2017. PMID: 28190065 Free PMC article.
-
Evaluating the performance of a rapid antigen test for the detection of influenza virus in clinical specimens from children in Cameroon.Influenza Other Respir Viruses. 2014 Mar;8(2):131-4. doi: 10.1111/irv.12210. Epub 2013 Nov 24. Influenza Other Respir Viruses. 2014. PMID: 24266902 Free PMC article.
-
Single Domain Antibodies as New Biomarker Detectors.Diagnostics (Basel). 2017 Oct 17;7(4):52. doi: 10.3390/diagnostics7040052. Diagnostics (Basel). 2017. PMID: 29039819 Free PMC article. Review.
References
-
- Anonymous 2006, posting date CDC awards $11.4 million to develop new rapid diagnostic tests for avian influenza. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/media/pressrel/r061204.htm
-
- Anonymous 2011, posting date Guidance for clinicians on the use of rapid influenza diagnostic tests. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm
-
- Anonymous 2009. Swine influenza A (H1N1) infection in two children—Southern California, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:400–402 - PubMed
-
- Biggs C, et al. 2010. Performance of influenza rapid antigen testing in influenza in emergency department patients. Emerg. Med. J. 27:5–7 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

