Intracellular transport of recombinant adeno-associated virus vectors
- PMID: 22357511
- PMCID: PMC4465241
- DOI: 10.1038/gt.2012.6
Intracellular transport of recombinant adeno-associated virus vectors
Abstract
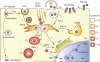
Recombinant adeno-associated viral vectors (rAAVs) have been widely used for gene delivery in animal models, and are currently evaluated for human gene therapy after successful clinical trials in the treatment of inherited, degenerative or acquired diseases, such as Leber congenital amaurosis, Parkinson disease or heart failure. However, limitations in vector tropism, such as limited tissue specificity and insufficient transduction efficiencies of particular tissues and cell types, still preclude therapeutic applications in certain tissues. Wild-type adeno-associated viruses (AAVs) are defective viruses that require the presence of a helper virus to complete their life cycle. On the one hand, this unique property makes AAV vectors one of the safest available viral vectors for gene delivery. On the other, it also represents a potential obstacle because rAAV vectors have to overcome several biological barriers in the absence of a helper virus to transduce successfully a cell. Consequently, a better understanding of the cellular roadblocks that limit rAAV gene delivery is crucial and, during the last 15 years, numerous studies resulted in an expanding body of knowledge of the intracellular trafficking pathways of rAAV vectors. This review describes our current understanding of the mechanisms involved in rAAV attachment to target cells, endocytosis, intracellular trafficking, capsid processing, nuclear import and genome release with an emphasis on the most recent discoveries in the field and the emerging strategies used to improve the efficiency of AAV-derived vectors.
Figures
References
-
- Kaneda Y. Update on non-viral delivery methods for cancer therapy: possibilities of a drug delivery system with anticancer activities beyond delivery as a new therapeutic tool. Expert Opin Drug Deliv. 2010;7(9):1079–93. - PubMed
-
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–72. - PubMed
-
- Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364(9452):2181–7. - PubMed
-
- Kohn DB, Candotti F. Gene therapy fulfilling its promise. N Engl J Med. 2009;360(5):518–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

