MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion
- PMID: 22357537
- PMCID: PMC5753594
- DOI: 10.1126/scitranslmed.3003441
MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion
Abstract
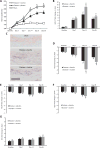
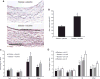
Identification and treatment of abdominal aortic aneurysm (AAA) remains among the most prominent challenges in vascular medicine. MicroRNAs are crucial regulators of cardiovascular pathology and represent possible targets for the inhibition of AAA expansion. We identified microRNA-21 (miR-21) as a key modulator of proliferation and apoptosis of vascular wall smooth muscle cells during development of AAA in two established murine models. In both models (AAA induced by porcine pancreatic elastase or infusion of angiotensin II), miR-21 expression increased as AAA developed. Lentiviral overexpression of miR-21 induced cell proliferation and decreased apoptosis in the aortic wall, with protective effects on aneurysm expansion. miR-21 overexpression substantially decreased expression of the phosphatase and tensin homolog (PTEN) protein, leading to increased phosphorylation and activation of AKT, a component of a pro-proliferative and antiapoptotic pathway. Systemic injection of a locked nucleic acid-modified antagomir targeting miR-21 diminished the pro-proliferative impact of down-regulated PTEN, leading to a marked increase in the size of AAA. Similar results were seen in mice with AAA augmented by nicotine and in human aortic tissue samples from patients undergoing surgical repair of AAA (with more pronounced effects observed in smokers). Modulation of miR-21 expression shows potential as a new therapeutic option to limit AAA expansion and vascular disease progression.
Conflict of interest statement
Figures




References
-
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: A best-evidence systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2005;142:203–211. - PubMed
-
- Creager MA, Jones DW, Easton JD, Halperin JL, Hirsch AT, Matsumoto AH, O’Gara PT, Safian RD, Schwartz GL, Spittell JA American Heart Association, Atherosclerotic Vascular Disease Conference: Writing Group V. Medical decision making and therapy. Circulation. 2004;109:2634–2642. - PubMed
-
- Powell JT, Worrell P, MacSweeney ST, Franks PJ, Greenhalgh RM. Smoking as a risk factor for abdominal aortic aneurysm. Ann. N. Y. Acad. Sci. 1996;800:246–248. - PubMed
-
- Lederle FA, Nelson DB, Joseph AM. Smokers’ relative risk for aortic aneurysm compared with other smoking-related diseases: A systematic review. J Vasc. Surg. 2003;38:329–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

